Biochemistry Review Topics
Students should review the following concepts from previous chemistry before the first class in CHEM 335 (Biochemistry I).
General Topics to Review (links are to outcome statements for previous courses, where available)
- Mathemathics
- Algebra (solving for a single variable)
- Fractions
- Logarithms
- Natural logarithm (ln)
- Base 10 logarithm (log)
- Generally how to work with them (+,-,/,*)
- Bonding
- Lewis dot structures and VSEPR theory
- Electronegativity
- Polarity of chemical bonds and molecules
- Intermolecular interactions
- Van der Waals
- Hydrogen bonding
- Thermodynamics
- Equilibrium Constants
- Acid-Base Chemistry
- pH
- pKa
- Titration curves
- Polyprotic acids
- Biology
- Eukaryotes
- Prokaryotes
- Organic Chemistry
- Line drawings
- Fischer projections
- Functional groups
- Hydrophilicity
- Hydrophobicity
- Mechanisms
- How you write them (equilibrium arrows, nonequilibrium arrows, drawing arrows from electrons to the atom where they are going)
- SN2-Hydrolysis (acid catalyzed and base catalyzed)
- Aldol condensation
- Carbonyl chemistry
Some Specific Review Concepts
- Electronegativity
- Electronegativity is a measure of the ability of an atom in a molecule to accept electron density. The larger the electronegativity value, the greater the ability an atom has to attract electron density. Specific electronegativity values were defined by Pauling. Electronegativity values increase from left to right across the periodic table and from the bottom up.
- Example: O is more electronegative than C, F is more electronegative than Cl
- Polarity
- The degree of polarity in a bond, or the difference in electron density between the atoms, can be determined from the difference in electronegativity values.
- Example: polarity of bond: C�H < C�O < C�F
- Intermolecular Forces
- van der Waals Forces
- Dipole-Dipole Interactions
- Exist between neutral polar molecules
- London Dispersion Forces
- Exist between neutral nonpolar molecules
- Relies on the polarizability of electron density
- Example: CH3(CH2)3CH3 and C6H6
- Dipole-Dipole Interactions
- Hydrogen Bonding
- Exists between a hydrogen atom in a polar molecule and a lone pair in an electronegative atom
- Need a hydrogen-bond donor (two atoms; one electronegative and one H)
- Need a hydrogen-bond acceptor (an electronegative atom with a lone pair)
- Electronegative atoms involved in H-bonding: O, N, F, sometimes Cl
- A dotted line indicates a H-bond Examples: N�H—-O, O�H—N, O—-H�F
- Ion-Dipole Interactions
- Occurs between an ion and a polar portion of a molecule
- Example: C=O and Na+Functional Groups/Classes of Compounds
- van der Waals Forces
| R�OH | Hydroxyl group/alcohol |
|---|---|
| R�NH2 | Amine |
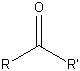 | Ketone (carbonyl) |
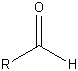 | Aldehyde |
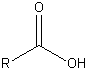 | Carboxylic acid |
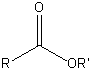 | Ester |
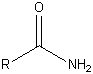 | Amide |
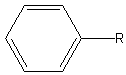 | Phenyl |
Logarithms
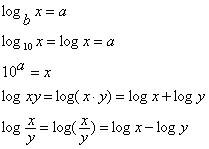 |
Acid/Base Chemistry
1) pH
pH = -log [H+]
pH is a measure of the acidity or # of H+ ions in solution
pH < 7 acidic pH = 7, neutral pH > 7 basic
2) pKa
pKa = -log Ka
When pH = pKa, one-half of the species are protonated and one half are not.
Example: If pKa = 5 and there are 10 molecules, then at pH = 5 there are 5 molecules with the H+ and 5 without
Dr. Nagan’s Informal Rules to Writing Mechanisms
1) Negative things attack positive things,
2) Start from the most negative atom (look for a nucleophile or base),
3) Electrons move in the direction of the arrow,
4) Follow your arrows to see what happens next,
5) If you added something, you usually have to have something leave or rearrange,
The best leaving group is very stable after it has left.
6) Make sure formal charges and valencies of atoms are correct.
Mechanism Problems
For each of the reactions, below:
a) draw the product(s), and
Last Update August 22, 2011