Outcome Statements for Chemistry 131: Chemical Principles II
Last Update: November 25, 2013
At the end of Chemistry 131 a student will have the following skills and knowledge (grouped by topic). Note that the number of outcome statements is not necessarily related to the amount of lecture time spent on a topic. An * indicates a topic covered in laboratory. These outcome statements assume that a student has successfully met the outcomes for CHEM 130.
Electronic Structure of the Hydrogen Atom
- •Describe the following and explain their historical significance: the ultraviolet catastrophe (and Planck’s solution), the photoelectric effect (use equation for), deBroglie’s equation (and be able to use), the Heisenberg uncertainty principle, Bohr’s model of the atom, the Rydberg equation (and be able to use)
- •Convert between energy and wavelength/frequency using E = hn and ln = c
- •Explain the term wavefunction and how this relates to the electron density and the probability of finding an electron at a certain position in space
- •Explain the term operator, and how it relates to quantum mechanics; give an example of an operator
- •Recognize that, in the quantum mechanical model, the electron’s angular momentum as it “orbits” the nucleus is quantized
- •Explain what a quantum number is, what it describes and give the possible values for the three quantum numbers that come from the interaction of the electron with the nucleus
- •Tell which quantum number defines the energy of the electron in the one-electron model and use the expression for the energy in terms of this quantum number to calculate wavelength/frequency of light emitted/absorbed by a one-electron atom
- •Define the terms excited state and ground state, and recognize for the hydrogen atom
- •Be able to draw qualitative radial wavefunctions, probability profiles and radial distribution functions for s, p and d orbitals (use these to explain penetration effect and shielding in the multi-electron atom)
- •Qualitatively draw the angular wavefunctions for s, p and d orbitals, differentiating between the different p and d orbitals and appreciating the directed nature of the electron density vis-à-vis bonding
- •Understand that the model for the multi-electron atom is a modified version of the hydrogen atom with corrections for electron-electron repulsion and exchange
- •Understand that electron spin must be taken into account in the multi-electron atom and that it is the intrinsic angular momentum of the electron
- •Understand that as a consequence of electron spin, only two electrons can occupy an orbital (Pauli Exclusion Principle), and that this limits the possible quantum numbers an electron in a multi-electron atom can have
- •Use the Pauli Exclusion Principle to give the correct set of four quantum numbers for any electron in a multi-electron atom
- •Appreciate that there is a difference between a one-electron orbital occupation diagram and multi-electron state
- •Appreciate that, with more than one-electron (in the absence of a magnetic or electric field), the orbital energy depends on two quantum numbers
- •Define the terms shielding, effective nuclear charge and penetration
- •Use graphs of the radial wave functions to explain orbital energy differences arise from shielding/effective nuclear charge and penetration
- •Explain the observed elemental electronic configurations in terms of the orbital energy, the spin-pairing energy and exchange interactions
- •Appreciate that Hund’s Rules are empirical rules for predicting ground state electronic configurations, use them to predict the electronic ground state configuration of neutral atoms and monatomic ions using the Aufbau Principle using both the electron-box and spectroscopic notation formalisms
- •Distinguish ground and excited state electronic configurations of atoms and ions
- •Recognize that the half-filled and completely filled subshell configurations are particularly stable electronic configurations in many cases
- •Explain how the concept of valence electrons arises from the quantum mechanical model of the multi-electron atom
- •Differentiate between core and valence electrons
- •Use the electronic structure to predict what ions a particular element will form, relate this to observed chemistry of the elements
- •Define the following terms: atomic radius, ionic radius, electron affinity, ionization energy and electronegativity
- •Be able to explain group and periodic trends (or lack thereof) in atomic radii, ionic radii, electron affinities, ionization energies and electronegativities using quantum mechanical multi-electron atom model
- •Define and explain the terms paramagnetism, diamagnetism and ferromagnetism
- •Recognize that there is a systematic change in the number of unpaired electrons, and therefore magnetic behavior, across a period
Lewis Dot Structure, VSEPR Theory and Valence Bond Theory Approaches to Bonding
- •Understand that only some electrons (valence electrons) participate in bonding while others (core electrons) do not
- •Determine the number of valence electrons for a main group element from its position in the periodic table
- •State the octet rule and explain why some elements are allowed to have an expanded octet
- •When presented with a formula or simple structural formula for a molecule or polyatomic ion, be able to draw a Lewis dot structure (including expanded octets and resonance structures), VSEPR structure, determine the hybridization of any atom in the molecule, and predict the actual structure
- •Understand that Lewis dot structures and valence bond theory are localized bonding models
- •Define formal charge, and be able to calculate it from a Lewis dot structure
- •Explain the difference between a formal charge and an oxidation number; be able to determine an oxidation number from a Lewis dot structure
- •Explain electronegativity and how that affects charge distribution in a bond
- •Explain the term resonance and why it must be invoked in Lewis dot structures; be able to draw resonance structures and resonance hybrids
- •Explain the basis of VSEPR theory (electrons take up space and distance between electron density is maximized)
- •Use VSEPR theory and Lewis dot structures to explain molecular properties such as bond lengths, bond angles, dipole moments and bond order/character
- •Explain the basis of valence bond theory and why hybridization of atomic orbitals is necessary
- •Explain the difference between σ and π bonds in valence bond theory
- •Understand the nature of resonance within the valence bond model
- •Draw and name the hybrid orbitals that correspond to each of the VSEPR electron pair geometries
- •Use bond enthalpies to estimate enthalpies of reactions, and explain why these ΔHrxn are only estimates
- •Explain the rationale behind molecular orbital theory (linear combination of atomic orbitals)
- •Understand that an electron is not localized in MO theory
- •Understand that MO theory is often too complex to apply in everyday situations, and simplified approaches (Lewis dot structures, VSPER, valence bond theory) are required
- •Understand that MO theory is a delocalized model of bonding (electrons are not required to be on only one atom) and that orbitals must overlap for bonds to form
- •Define the following: bond length, bond angle, bond polarity, dipole moment, bond order, bond character in terms of MO theory
- •Define the terms s/p bonding orbital, s/p anti-bonding orbital and non-bonding orbital, and be able to draw simples/p bonding and anti-bonding orbitals
- •Explain the difference between s and p bonds in valence bond theory as compared to MO theory
- •Understand that in MO theory the strength of a bond depends on the overlap of the atomic orbitals (no overlap, no bond) and the energy difference between the orbitals (large energy difference, weak covalent bond), relevance to strength of bonds within a group and the formation of an ionic bond
- •Appreciate that bond character is a continuum (covalent®polar, covalent®ionic)
- •Know that electronegativity is a rough estimate of orbital energy differences and explain qualitatively how that changes an MO diagram
- •List the important physical properties that define the solid state
- •Know the five basic types of solid substances, the bonding involved in each and their typical properties
- •Explain how bonding in the solid state (lattice energy and covalency) affects the properties of the substance (solubility, hardness, melting point, conductivity)
- •Describe how atoms and molecules are arranged in the solid state, define unit cell
- •Identify some of the Bravais lattice types (primitive, body-centered, face-centered and noncubic types)
- •Understand how diffraction is applied to determine X-ray crystal structures and appreciate the process involved in solving crystal structures
- •Determine the number of ions in a unit cell
- •Calculate the density of a particular lattice type (e.g. closest packed solid)
- •Understand the difference between ionic, molecular and atomic solids
- •Understand how Coulomb’s Las effects the interaction energy between two ions
- •Determine a chemical formula from a picture of the unit cell
- •Understand the difference between graphite and diamond
- •Understand how atoms are arranged in a solid metal (hcp, ccp)
- •Use close packing to describe metals
- •Understand that in metallic substances, valence electrons are shared.
- •Understand and explain the band model or molecular orbital model for metals
- •Compare and contrast ionic bonding within the Lewis dot and MO formalisms
- •Explain what the lattice energy is and its relationship to the properties of ionic compounds
- •Calculate the lattice energy using the Born-Haber cycle
- •Recognize the seven common lattice types for ionic compounds (sphalerite, wurtzite, sodium chloride, cesium chloride, rutile, fluorite, antifluorite)
- •Describe these lattices types in terms of hcp and ccp models with different ions packing and occupying either octahedral or tetrahedral holes
- •Define conductor and semiconductor, explain how they differ
- •Know the definitions of the following: defect, solution alloy, heterogeneous alloy, intermetallic compound
- •Understand that condensed states include both liquids and solids
- •Define intermolecular forces and understand that molecules held together by these forces remain intact
- •Define and differentiate between ion-dipole, dipole-dipole, dipole-induced dipole, induced dipole-induced dipole, hydrogen bonding and London dispersion forces.
- •Define surface tension, viscosity and capillary action
- •Describe the relationship between polarity and the strength of intermolecular forces
- •Define vaporization, condensation, sublimation and fusion
- •Define enthalpies of transition such as ΔHvap, ΔHsub, ΔHfus
- •Define vapor pressure use it to explain when boiling occurs
- •Rationalize trends in physical properties such as boiling point, freezing point and vapor pressure using intermolecular forces
- •Describe the phase transitions in a simple heating curve (e.g. for water)
- •Understand the terms superheating and supercooling and some practical applications
- •Draw a phase diagram for a molecule
- •Identify the boiling point, melting point, freezing point, triple point critical point on a phase diagram
Properties of Solutions with Colligative Properties
- •Define units of concentration (given in CHEM 130 outcomes)
- •Define solubility
- •Understand that “like dissolves like” (polar solvents dissolve polar solutes, nonpolar solvents dissolve nonpolar solutes)
- •Understand the difference between hydrophobic and hydrophilic molecules
- •Understand the factors that affect solubility such as structure (lattice energy, intermolecular interactions), temperature and pressure
- •Define chromatography and give examples of different chromatographic methods
- •Explain how intermolecular interactions allow for separation of materials in solution by chromatography
- •Define ΔHsoln
- •Understand that the ΔHsoln is made up of three components: the energy to separate both the solute and solvent molecules as well as the interaction energy associated with the solute and solvent associating
- •Use Henry’s law to calculate the concentrations of gas dissolved in a solvent
- •Use Raoult’s law to calculate the vapor pressure of a solution
- •Calculate the vapor pressure of a solution containing two liquids
- •Calculate molar mass, and boiling-point elevation based upon
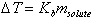
- •Calculate molar mass and freezing-point depression based upon
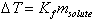
- •Calculate molar mass and osmotic pressure based upon
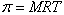
- •Understand why molality and not molarity is used in the freezing-point and boiling-point equations
- •Understand that there is finite amount of solute that can be dissolved in a solvent. Understand this concept in terms of equilibrium.
- •Understand the terms saturated and supersaturated solutions in terms of kinetics
- •Understand the terms miscible and immiscible in terms of intermolecular forces
•Know the definitions of electrolyte (strong and weak) and non-electrolyte, relate these to the equilibria involved
•Be able to write net ionic equations
•Apply thermodynamics, kinetics and stoichiometry to these systems
- •Know and explain different definitions of acids and bases (Arrhenius, Brønsted-Lowry, Lewis)
- •Know the definition of Ka and Kb
- •Explain what strong and weak acids/bases are and the relationship to the equilibrium constant (Ka or Kb)
- •Explain why metal oxides are basic, non-metal oxides are acidic
- •Explain the terms amphiprotic and polyprotic
- •Define Kw and explain where it comes from, know when Kw must be accounted for in a calculation
- •Explain what a conjugate acid-base pair is and how this may be used to qualitatively predict the relative strengths of an acid and base
- •Define and explain pH and the pH scale (know what pH values are acidic and which are basic)
- •Be able to calculate pH from Ka/Kb and calculate Ka/Kb from pH
- •Predict the pH of a solution arising from hydrolysis of a salt
- •Perform pH calculation with polyprotic acids or bases
- •Describe the reactions of strong acids/bases, strong acid/base with weak base/acid, weak acids/bases; indicate which will have a pH of 7.00 at the equivalence point and why
- •Common ion effect in relationship to weak acid/base equilibria
- •Definition of a buffer
- •Use the Henderson-Hasselbalch equation to predict the pH of a buffer and to find the concentration of the acid/base needed to prepare a buffer of a given pH
- •Derive acid-base titration curves for any combination of strong and weak acid/base
- •Define “insoluble” as very slightly soluble ionic compounds
- •Know general solubility rules
- •Be able to write products for simple precipitation reactions based on solubility rules and Ksp values
- •Explain solubility rules for ionic compounds in terms of the interactions occurring at the molecular level
- •Define Ksp
- •Use a table of Ksp values properly
- •Define the ion product, Q, and understand the difference between Q and Ksp
- •Determine Ksp from experimental measurements
- •Estimate salt solubility from Ksp
- •Understand that solubilities are expressed in mol/L in Ksp calculations
- •Appreciate that the proper thermodynamic expression for Ksp requires activities and that the use of concentration is an approximation
- •Understand that relative solubilities of salts can only be made using Ksp when the number of total ions is the same
- •Use Ksp to determine precipitation conditions
- •Use Ksp to determine the concentration of ions in solution
- •Use Ksp to describe precipitation of insoluble salts
- •Explain what a common ion is and use the common ion effect appropriately in any of the calculations described above
- •Understand when and how to make the approximations in Ksp calculations that reduce cubic equations to quadratic equations (5% rule)
- •Use solubility properties to determine the identity of an unknown solution (qualitative analysis)
- •Understand when pH may affect an equilibrium equation and modify the solubility calculation appropriately
- •Use Ksp values to selectively precipitate one species over another
- Redox Reactions
-
- •Define oxidation, reduction, oxidizing agents and reducing agents
- •Define and use half-reactions
- •Balance redox reaction in acidic, basic or neutral media
- •Understand how a galvanic cell works
- •Understand what a cell potential, voltmeter and potentiometer are
- •Use standard reduction potentials to calculate cell potentials (E°cell)
- •Use line notation for a galvanic cell
- •Understand the relationship between free energy and the cell potential
- •Use the Nernst equation to predict cell potential and use it to find K
- •Understand how ion-selective electrodes work
- •Understand how batteries work
Transition Metal Chemistry and Coordination Chemistry
- •Define a complex ion, a coordination compound, coordination number and a counter ion
- •Explain why coordination chemistry is Lewis acid/base chemistry
- •Be able to identify the transition metal and its ligands
- •Be able to identify monodentate (NH3, CN–, SCN–, etc.), bidentate (en, oxalate, etc.), and polydentate ligands (EDTA, dien, etc.)
- •Know the difference between stepwise and cumulative formation constants, be able to convert between the two
- •Name coordination compounds
- •Recognize and name common geometries of coordination compounds
- •Review isomers but in context of transition metal complexes
-
- ¤Distinguish between structural isomers and stereoisomers
- ¤Identify diastereomers and enantiomers
- ¤Use and understand isomer nomenclature (mer-, fac-, Λ-, Δ-)
- •Understand bonding in complex ions
-
- ¤Explain the basis of the crystal field model
- ¤Predict d-orbital splitting based on crystal field theory
- ¤Understand that the magnetism of metal ion-containing compounds depends on the number of electrons and the d-orbital splitting
- ¤Explain the origin of high-spin and low-spin complexes in terms of the spin-pairing energy and the d-orbital splitting
- ¤Understand that the spectrochemical series arises from how the ligands affect the splitting of the d orbitals
- ¤Understand all of the above for octahedral and tetrahedral complexes
- •Define the terms: alpha particle, beta particle, gamma emission, and positron
- •Be able to explain the modes of radioactive decay (alpha, beta, gamma and positron emission, electron capture)
- •Write equations for nuclear reactions
- •Explain what a radioactive decay series is, define the term daughter isotope
- •Understand the rules for nuclear stability and use them to predict modes of decay for unstable nuclei
- •Appreciate the uses of nuclear processes in energy production, medicine, etc.
- •Define the term liquid crystal and the terms nematic, smectic, cholesteric liquid crystalline phase (be able to draw pictures of these different crystalline phases)
- •Recognize what molecular features lead to liquid crystalline behavior
- •Define terms polymer and monomer; give examples of each
- •Recognize polymerization can occur through addition polymerization or condensation polymerization
- •Explain how the different types (thermoplastic, thermosetting and elastomer) of plastics are different in terms of their physical properties (crystallinity, density, stiffness, melting point)
- •Explain cross-linking and how it affects polymer properties
- •Explain what a ceramic is; give an example of a ceramic
- •Describe methods of ceramic preparation (sol-gel process, sintering, composites)
- •Define piezoelectric, explain why it is important and give an example of a piezoelectric material
- •Define the terms superconductors, superconductivity and critical temperature; give examples of high Tc superconductors
- •Define the term thin film, explain its importance, and give the methods (sputtering, vacuum deposition, chemical-vapor deposition) for forming thin films
- •Explain what colloids, gels, sols, micelles, surfactants and emulsions are, how they form and give examples of how they are used
