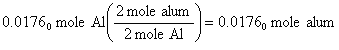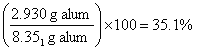Calculating the Percent Yield of Alum
Last Update: April 25, 2011
To determine the percent yield of a product in a chemical reaction we need to know the amount of all reactants used, the amount of the product formed and the balanced chemical reaction. From the balanced chemical reaction and the amount of reactants, we determine first the limiting reagent and then theoretical yield of the product. The percent yield is then simply the actual amount of product obtained divided by the theoretical yield times 100.
For the reaction of Al with KOH to form alum the balanced chemical reaction is as follows:
2 Al (s) + 2 KOH (aq) + 22 H2O (l) + 4 H2SO4 (aq) → 2 KAl(SO4)2 + 12 H2O (s) + 3 H2 (g)
To simplify things we have told you that the Al is the limiting reagent (if you wish, you can check this). Since we do not need to determine the limiting reagent, our first step is to determine the amount of alum that can theoretically be formed from the amount of Al that we have. Let’s assume that we used 0.475 g of Al and that we obtained 2.930 g of alum. Note that throughout this page extra insignificant figures carried along in the calculation to prevent rounding errors.
First, we must determine the moles of Al in 0.475 g of Al. You should get 0.01750 moles of Al (Help Me).
Now convert moles of Al to moles of alum using the stoichiometric factor from the balanced chemical equation. You should have found that the reaction could form 0.01750moles of alum (Help Me).
Calculate the mass of alum (in grams) from moles of alum. This is the theoretical yield. CAUTION! the molar mass of alum includes K, Al, S and O and the twelve H2O! You should get 8.351 g of alum (Help Me).
Determine the percent yield. Your result should be 35.1% to the correct number of significant figures, although this would often only reported as 35% (Help Me).
The calculation from the mass of Al to the mass of alum could be done in a single step (Help Me).