Preparation and Analysis of Alum1
Authors: D. L. McCurdy, V. M. Pultz and J. M. McCormick*
Last Update: August 21, 2014
One of chemistry’s goals is to be able to transform any set of substances (the reactants) to another set of substances (the products) through a chemical reaction. As we have discussed in class, there are rules, such as the Law of Conservation of Mass, by which chemical reactions occur, and it took chemists a long time to understand these basic rules. Even though we know a great deal about chemical reactions, chemists are still finding new chemical reactions and new ways of assembling atoms into molecules and molecules into more elaborate structures. In this and the next laboratory exercise you will learn some of the basics of how chemists carry out chemical reactions and how they characterize the chemical substances involved in these reactions.
To fully describe a chemical reaction one needs to know the identities of both the products and the reactants, and the proportions in which the reactants combine and the products form. While it may seem a trivial exercise to identify the reactants, this is not always the case. Needless to say, identification of the reactants in a complex reaction mixture can be very difficult, and so we will only work with chemical reactions where the reactants are known.
The description of a chemical reaction consists of a series of steps: 1) carrying out the reaction, 2) isolating the product(s), 3) purifying the product(s), 4) and characterizing the product(s) and determining its(their) purity. The isolation and purification of the products are based on physical properties such as the ability to form crystals, boiling point, melting point, solubility, etc. Characterization of the products may be either quantitative or qualitative. In a quantitative characterization, the chemical formula and the structure (i. e., how the atoms are connected) are determined. The former is usually accomplished using elemental analysis, mass spectroscopy, X-ray crystallography or some spectroscopic method. Sometimes it is sufficient to show only that certain ions or elements are present in a sample, and in this case a chemist will perform a qualitative test. Qualitative tests often use chemical reactions that result in a visible change (formation of an insoluble solid, a color change, or evolution of a gas) as a way to quickly show whether a particular chemical species is present or not.
Once the chemical reaction’s products are fully characterized, and the balanced chemical reaction is known, we can compute a theoretical and a percent yield. We do these final characterizations of the reaction because it is important to know how efficiently the reaction converts reactants to products. Chemists are always trying to strike a balance between the cost of the reactants, the value of the products, the time a reaction requires and the cost of any unwanted by-products that must be handled as hazardous waste. A reaction, even though it gives a valuable product, may be unusable because it has a low yield, takes too much time or generates too much waste.
In this experiment you will prepare and characterize alum (potassium aluminum sulfate dodecahydrate, KAl(SO4)2·12 H2O). The first step in this synthesis, which you will perform during Week 1, is to react metallic aluminum with a concentrated solution of potassium hydroxide (KOH) to form the potassium salt of the tetrahydroxoaluminate complex ion, [Al(OH)4]–. The balanced chemical equation for this oxidation-reduction reaction is
![]()
The second step of the procedure is to convert the KAl(OH)4 to alum by addition of sulfuric acid (H2SO4) in an acid-base reaction. Under the experimental conditions, the alum has a limited solubility in water, and so it precipitates from the solution. The balanced chemical reaction that occurs in this step is
![]()
The overall balanced chemical reaction for the conversion of aluminum to alum, shown below, can be obtained by adding together the balanced chemical equation for each step (Help Me).
![]()
The second and third weeks of this exercise will be devoted to characterizing the alum. Alum is an ionic compound, which means its melting and boiling points are likely to be too high to be measured conveniently. Also, most spectroscopic methods would not yield useful information. Therefore, we will rely on chemical means to show that we did, in fact, form alum in our reaction. This procedure duplicates how chemists characterized chemical reactions until the late 20th century, and in some cases chemical means of characterization are still the only methods available.
In Week 2 you will perform qualitative tests to demonstrate the presence of K+, and sulfate ion (SO42-) in the alum. You will also perform a quantitative determination to determine the percent water by mass in alum.
The qualitative test for sulfate uses the insolubility of barium sulfate (BaSO4). When an aqueous solution of a barium salt (usually BaCl2) is mixed with an aqueous solution containing sulfate, a white precipitate of insoluble BaSO4 forms according to the net ionic equation: Ba2+ (aq) + SO42- (aq) → BaSO4 (s). A positive test for SO42-, therefore, is the observation of a white precipitate when an aqueous BaCl2 solution is mixed with the aqueous test solution.
When placed in a flame many elements give the flame a distinctive color; an effect that can be used to determine both which elements, and how much of each one, is present in a sample. Potassium produces a distinctive lavender flame that we can use as a qualitative test for the presence of potassium. Potassium’s flame is often difficult to see because sodium, which is often present as an impurity, has an intense yellow flame that masks other colors. The potassium flame can be seen in the presence of sodium by viewing the flame through a dark blue cobalt-glass filter, which absorbs the yellow light from Na, but allows the light from K to pass. When placed in a flame, aluminum does not change the flame’s color, and so a visual flame test cannot be used to show the presence of Al.
Alum is a hydrate, which means that it is a compound that has water molecules trapped within the solid. Hydrates will release some, or all, of their “waters of hydration” upon heating. If the chemical reaction between Al and KOH does produce alum as a product, we would expect that heating the product should result in a decrease in the sample’s weight corresponding to the loss of 12 water molecules per formula unit of alum. Thus, if one knows the starting mass of alum, and the amount (mass, and therefore number of moles) of anhydrous alum remaining after all of the water has been driven off, one can calculate the amount of water that was present in the alum (by the Law of Conservation of Mass). A comparison of the experimentally determined waters of hydration and the number expected from the chemical formula can then be used as evidence for the formation of the desired product. The process by which the waters of hydration are driven off is described by the chemical equation shown below, where the “Δ” written above the arrow indicates that heat was applied to the reactant(s).
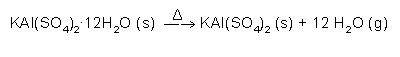
A quantitative analysis for Al3+ will be made in Week 3. Normally, Al3+ is colorless, which means that it does not absorb light in the visible portion of the spectrum. So, we will add a dye called aluminon that will react with the Al3+ in solution to give a colored complex ion. For a sufficiently dilute solution, the amount of light absorbed by a chromophore (a chemical species that absorbs light) present in the solution is given by Beer’s Law, A =ε•b•C, where A is the absorbance (how much light the sample absorbs compared to a solution that does not contain the chromophore), ε is the molar absorptivity (also known as an extinction coefficient; ε depends on the compound and the wavelength of light), bis the pathlength (how much sample the light must pass through) and C is the concentration of the chromophore (More Info). According to Beer’s Law the intensity of the color is linearly dependent on the amount of aluminon-Al3+ complex present. So if we knew ε for the complex ion formed between Al3+ and aluminon, we could make a single absorbance measurement and know the [Al3+] in a solution, and therefore, how much Al was in the original alum sample. Unfortunately, this is neither a precise nor accurate way to make this determination. It is imprecise because it is only a single measurement, and it is inaccurate because 1) we don’t know the stoichiometry for the reaction between aluminon and Al3+ and 2) the commercially available dye is not pure (ε cannot be determined). So, we need a way to increase the method’s precision and to overcome the problem with accuracy.
The problems with the colorimetric method are solved by using a calibration curve, which gives the relationship between absorbance and concentration. A calibration curve is constructed by preparing samples with known concentrations of the analyte (in our case, Al3+) and then measuring the absorbance of these samples. If Beer’s Law holds, a calibration curve is a straight line, for which we can obtain an equation from a regression analysis. Now if we measure the absorbance of a sample containing an unknown amount of analyte, it becomes a simple matter of substituting this value into the equation for our calibration curve and solving for the concentration. Because more than one measurement was used to construct the calibration curve, we improve our precision. A calibration curve also improves accuracy because only the analyte’s concentration changes (everything else, such as the stoichiometry between aluminon and Al3+ and the dye’s purity, is constant).
When you set up your laboratory notebook for this exercise treat each week of the exercise as a separate experiment. So, each week will have its own title, statement of purpose, etc. Note that some of your results will actually be determined during a subsequent week. Be sure to carefully read the experimental procedure and be aware that there are a number of potential hazards. Also, there are several places in this exercise where you will be waiting for something to happen. You can substantially shorten your time in lab by working on another section of that week’s exercise during these times. Also, be sure that you have completed all of the calculations for a given week’s work before coming to laboratory. If you do not come to laboratory prepared, you will not be able to complete the Week 2 and Week 3 exercises in the allotted time.
Week 1 ( More Info)
Synthesis of Potassium Aluminum Sulfate Dodecahydrate
Obtain a piece of aluminum foil weighing about 0.5 g and weigh it precisely (to the nearest 0.001g). Cut the weighed foil into many small pieces. The smaller the pieces the faster the reaction will go because of the increased surface area exposed to the KOH solution.
Place the small pieces of aluminum in a 100-mL beaker. Add enough hot water to a Styrofoam cup so that, when the 100-mL beaker is placed inside, the beaker is completely surrounded by water, but the water does not spill out of the cup or into the beaker. If water from the hot water bath spills into the beaker there will be a drasticdecrease in the yield of alum.
Place the 100-mL beaker containing the aluminum into the hot water in the Styrofoam cup and transfer everything to the hood. Slowly and carefully add 25 mL of the 1.4 M KOH solution to the aluminum. CAUTION! No open flames can be present in lab while the reaction between KOH and Al is taking place. Stir the solution with your glass stirring rod and cover it with a watch glass. Repeat the stirring every few minutes until all of the aluminum dissolves. If the reaction slows down, replace the water in the bath with fresh hot water. If the reaction becomes too vigorous, remove the beaker from the water bath until the reaction subsides. CAUTION! Avoid inhaling the gas evolved during this reaction. The gas is not toxic at this concentration, but a fine mist of the corrosive KOH solution is formed by the gas evolution.
When the aluminum has completely dissolved (do not be concerned if the solution appears cloudy or contains black specks), gravity filter the reaction mixture into a 50-mL beaker through fluted filter paper (the instructor will demonstrate). Dispose of the used filter paper in the laboratory garbage. CAUTION! The filter paper will be wet with the corrosive KOH solution. So, wash your hands after handling the wet filter paper.
Obtain approximately 5 mL of 9 M H2SO4 in your 10 mL graduated cylinder. Use a plastic pipet to slowly and carefully add the H2SO4 solution to the 50-mL beaker containing your filtered solution. Do not dip the pipet into the filtered solution! Continue adding the H2SO4 solution until no more precipitate forms. This should require no more than about 5 mL of the H2SO4 solution. Do not add too much H2SO4, or your yield will suffer (More Info). After the H2SO4 addition, carefully stir the new mixture with your stirring rod and record your observations. CAUTION! The H2SO4 solution is very corrosive and the reaction between the H2SO4 and KOH is very exothermic (gives off heat).
Prepare a cold bath. Place the 50-mL beaker with the filtered reaction solution in the ice bath. Do not introduce any of the water from the ice bath into the beaker. Also place a test tube containing 15 mL of 95% ethanol in the ice bath. The ethanol solution will be used to wash residual H2SO4 from the alum crystals.
After a crop of crystals has formed, set up a vacuum filtration apparatus (Help Me). Do not under any circumstances push the rubber tubing more than 1/4” on to the side-arm of the filter flask and do not leave the tubing attached to the flask while the flask is unclamped.
While the vacuum is on, carefully remove some of the supernatant (the solution above a solid) from your crystals using a pipette and wet the filter paper. This will help the paper adhere to the filter and prevents leaks. CAUTION! The solution is corrosive. Remove the 50-mL beaker from the ice bath, swirl it gently to suspend the crystals and pour it into the Büchner funnel. Use your glass stir rod to remove any crystals that adhere to the side of the beaker. Once the aqueous solution has been filtered completely (leaving the crystals on the filter paper), place 2 – 3 mL (the plastic pipets hold about 3 mL) of the cold ethanol solution in the 50-mL beaker. Use your glass stirring rod to loosen any remaining solid that clings to the side. Swirl to suspend any crystals remaining in the beaker, and pour the suspension into the filter. Once the ethanol has been filtered away, repeat this washing several times. After the last ethanol wash, allow the vacuum to run for a minute or two to draw air through the crystals to help them dry.
After the ethanol solution has stopped draining from the funnel, inspect the product. If it looks dry, gently prod it with your metal spatula. If it is dry enough to remove from the filter, the solid will not be very sticky and will have the consistency of fine sand. Break the vacuum by removing the vacuum hose from the side-arm of the filter flask, and then turn off the aspirator. Transfer the solid and the filter paper from the funnel to a pre-weighedwatch glass with the help of your metal spatula, as your instructor will demonstrate. Carefully scrape any alum that adheres to the side of the Büchner funnel onto the watch glass.
If the alum is dry, the filter paper will separate from the crystals and you can remove the filter paper. Gently scrape any crystals adhering to the filter paper onto the watch glass. If the alum is still too wet, leave the filter paper and remove it next week.
Obtain the mass of the wet alum. You will need to have about 2 g of wet alum (3 g if the mass includes the filter paper) so that you will have enough for the next two weeks. If you don’t have enough, collect the second crop of crystals and/or redo the synthesis. Keep the crystals from different crops and syntheses separate. Cover the container holding the crystals with a piece of paper towel, and place it in your drawer to dry.
You may notice that more crystals formed in the filter flask during the washings. This second crop of crystals may also be collected, but if you choose to collect these crystals, they should be kept separate from the main crop. It is always good laboratory practice to keep different crops of crystals separate until the identity and purity of each crop is determined (second crops almost always contain more impurities than the first crop and the time needed to purify them sometimes far outweighs the additional yield). Collect the second crystal crop by vacuum filtration; wash with several small portions of the cold ethanol solution and dry, as described above.
Before doing anything else in the laboratory obtain the mass of each crop of alum to the nearest milligram (three decimal places). Make observations on the crystalline product (color, texture, etc.), and record all of your observations in your laboratory notebook. Share your results with your classmates.
Qualitative Chemical Tests
Perform the following qualitative tests for SO42- and potassium on your sample. If you collected a second crop of alum crystals, you should perform the sulfate and potassium qualitative tests on both the first and second crops (are your two crops qualitatively the same?).
Sulfate Test
Place a few crystals of your alum in a 6” test tube. Add distilled water dropwise while stirring until the alum dissolves. Add one drop of 0.5 M BaCl2 (barium chloride). Record your results. Does alum contain sulfate?
Potassium Flame Test
The instructor will demonstrate the proper techniques for using the Bunsen burner and heating the needle. In the hood, heat the provided needle in the flame to remove impurities. Once the needle is clean, carefully scoop up a small amount of alum on the end of the hot needle. Place the alum in the flame and heat it until the crystals begin to melt and the solid glows. Note the color of the flame. If your flame is bright yellow (indicating the presence of sodium), try cleaning your needle again, or use the cobalt glass filter. Does this sample contain potassium?
Quantitative Determination of Waters of Hydration
Before beginning this section be sure that your alum sample is powdered and that you have weighed your alum sample!
Set up a ring stand, ring clamp and porcelain triangle, as your instructor will demonstrate.
Clean your crucible by placing a few drops of 1 M NH3 solution in the empty crucible and scrubbing with a paper towel. CAUTION! This ammonia solution has a strong odor and is corrosive. Rinse the crucible with distilled water and place the empty crucible on the porcelain triangle supported by a ring and ring stand.
With majority of the flame remaining below the bottom of the crucible, heat the crucible until its bottom glows a dull red. After heating for five minutes, remove the flame and let the crucible cool to room temperature on the triangle. CAUTION! Do not touch the crucible with your hand. It is extremely hot and will remain hot for several minutes. Remember that a hot crucible looks exactly the same as a cool crucible. When cooled, you can move the crucible to the bench top using the crucible tongs. Do not set a hot crucible on the bench top, because the temperature differential may cause the crucible to shatter. Once you have cleaned the crucible, it is important that you handle it only with the crucible tongs. This prevents burns and will eliminate a systematic error caused by the weight of your fingerprints.
Weigh the cooled crucible (and its cover) to the nearest milligram (three decimal places) and record this mass in your notebook. If the balance does not show three decimal places, notify the instructor. Place about 1.0 g of your alum sample in the crucible. Obtain the mass of the crucible, its cover, and the alum to the nearest milligram and record this in your notebook.
Return the crucible to the porcelain triangle and set the cover slightly ajar so that the water vapor can escape. For the first few minutes gently heat (only the light blue portion of the flame touches the crucible) the crucible by holding the Bunsen burner off to the side. Take care! The water can violently leave the alum at this point, if it is heated too strongly. Move the Bunsen burner such that the tip of the inner blue cone is approximately 3 cm below the crucible. Heat until the crucible glows red and continue heating for 10 minutes. If at any time you observe white smoke being given off, or smell an acrid odor, discontinue heating immediately (the sulfate is being decomposed to SO2).
Remove the heat and completely cover the crucible with the lid. Cool the crucible to room temperature on the triangle (this takes about ten minutes). Weigh the cooled crucible (including its cover and the contents) to the nearest milligram (three decimal places). Using the tongs, move the crucible and contents back to the triangle and repeat the heating step for 10 minutes. When this heating step is over, cover the crucible and allow it to cool on the triangle to room temperature, and then reweigh the crucible, cover, and its contents. Record this second mass in your notebook. If the second mass is within a 50 mg of the mass after the first heating, then you have driven off all of the water. If the masses are not within 50 mg, then repeat the heating procedure until two subsequent masses agree.
Once you have made your final weighing, invert the crucible and the anhydrous alum should fall out. If it does not, add some water from a squirt bottle and use your metal spatula gently to dislodge it. The anhydrous alum may be disposed of in the trash or in the sink with plenty of water. Rinse the crucible with distilled water and dry it before returning it to your drawer.
Before coming to the laboratory you must have completed the following: 1) prepare a table, like Table 1, in your notebook’s Results section in which to write your data for the calibration curve, 2) set up the calculations to calculate the [Al3+] in Table 1 (the number of significant figures in each volume is given in Table 1 and in Table 2), 3) prepare an Excel spreadsheet to graph the calibration curve (save on your Y: drive or a flash drive), and 4) familiarize yourself with the instrument before laboratory; your instructor will review spectrometer operations before you begin work (click here for operating instructions).
| Solution Number | Volume of Al3+ Stock Solution Used (mL) | Final Volume of Solution (mL) | [Al3+] (M) | Absorbance at 525nm |
|---|---|---|---|---|
| 1 | 0.00 | 50.00 | ||
| 2 | 1.00 | 50.00 | ||
| 3 | 2.00 | 50.00 | ||
| 4 | 3.00 | 50.00 | ||
| 5 | 5.00 | 50.00 |
Table 1. Example of a table that could be used to present the data for the calibration curve.
Your instructor will demonstrate how to prepare solutions using volumetric glassware and will review the protocols for using the balances.
Colorimetric Determination of Aluminum2,3
Preparation of the Aluminum Stock Solution
Precisely weigh out (to the nearest milligram) about 0.1 g AlCl3·6H2O using an analytical balance. Quantitatively transfer this solid to a 100-mL volumetric flask (assume the flask’s volume is 100.0 mL). Add about 10 mL of distilled water and swirl to dissolve the AlCl3·6H2O. If the solid does not dissolve, carefully add small amounts of distilled water, swirling between each addition, until it does. Add distilled water to bring the level of the solution in the flask to the mark on the neck (this procedure is called “diluting to the mark”). Mix thoroughly by stoppering the flask, and then inverting and shaking the flask. Repeat if necessary.
Pipet 3.00 mL of the aluminum solution that you just made into a 25-mL volumetric flask. Dilute to the mark and mix thoroughly. This is the aluminum stock solution that you will use to construct the calibration curve.
Construction of the Calibration Curve
Number five 50-mL volumetric flasks 1 to 5. Do not add any of the aluminum stock solution to flask 1. To flask 2 add 1.00 mL of the aluminum stock solution; to flask 3 add 2.00 mL; add 3.00 mL to flask 4 and 5.00 mL to flask 5. These measurements must be precise, and so you must use volumetric pipets.
To each flask then add 20 mL of the acetate buffer solution and 5 mL of the aluminon solution (in that order!) and swirl gently to mix. These volume measurements do not need to be highly precise. So, you can use your 50-mL and 10-mL graduated cylinders here. Dilute all the solutions to the mark by adding distilled water and mix thoroughly. Allow the solutions to sit for 20 min while monitoring the solutions’ colors. Note any changes in your notebook.
Follow the spectrometer’s operating instructions to ready the instrument for use. Fill the cuvette with the buffer solution to use as a blank (IMPORTANT! you must use the same cuvette for both the blank and for your samples). Remove any bubbles by gentlytapping the cuvette with your finger. Under absolutely no circumstances are you to tap a cuvette on a table top. Do not handle the cuvette by the clear window (your fingerprints will cause an error in the measurement). Before placing the cuvette into the spectrometer, be sure to thoroughly wipe the clear sides of the cuvette with a Kim-Wipe (do not use a paper towel). When placing the cuvette in the spectrometer, be sure that clear sides are aligned with the light beam and that the cuvette is placed in the spectrometer the same way every time. The major sources of error when using these spectrometers come from poor technique, and you can avoid these by following these guidelines every time you make a measurement with the spectrometer.
A representative spectrum of a solution of the Al3+:aluminon complex is shown in Figure 1. The spectrum should exhibit a broad peak near 525 nm. If the shape of your spectrum looks dramatically different than that in Figure 1, consult your instructor. Measure the absorbance at 525 nm (More Info) for solutions 1 through 5. Graph the absorbance at 525 nm as a function of [Al3+] in Excel (Help Me) and perform a linear regression of the data by inserting a trendline (Help Me) on the graph. Show your graph to your instructor; once he or she has approved it, you may proceed to the next section.
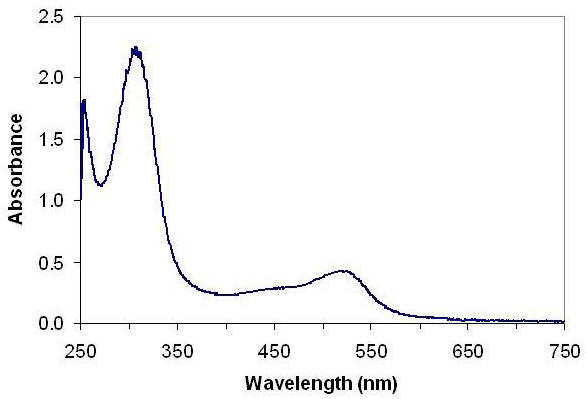
Figure 1. Representative absorbance spectrum for a dilute solution of the Al3+/aluminon complex in acetate buffer.
Determination of Aluminum in Alum
Precisely (to three decimal places) weigh out about 0.2 g of your alum. Quantitatively transfer, as your instructor will demonstrate, to a small beaker and add distilled water to bring the volume to about 15 mL. Place the beaker on a hot plate in the hood, cover with a watch glass and heat to boiling, stirring occasionally with your glass stirring rod. After stirring rinse the glass rod into the beaker with a small amount of distilled water from your wash bottle. While the mixture is heating, clean and dry (exterior only) your volumetric flasks. Also prepare for a gravity filtration directly into the 100-mL volumetric flask using a long-stemmed glass funnel.
Remove the beaker from the hot plate just as the solution starts to boil. CAUTION! The beaker, watch glass and the hot plate’s top are all hot. Your instructor will demonstrate the safe way to remove the beaker from the hot plate. Immediately pour the hot solution into the funnel. As the solution is being filtered rinse the beaker, the bottom of the watch glass and the stirring rod each with several small washes of distilled water into the funnel. When the solution is completely filtered, remove the funnel from the volumetric and rinse the volumetric’s neck with several small portions of distilled water. The volumetric should now be cool to the touch, but if it is not, wait until it is. Dilute the solution in the flask to the mark. Transfer 3.00 mL of this solution to the 25-mL volumetric flask and dilute as before.
Pipet 3.00 mL of the alum solution that you just prepared into a 50-mL volumetric. Add 20 mL of acetate buffer and 5 mL of aluminon solution using graduated cylinders and then dilute to the mark with distilled water. Wait 20 min and measure the absorbance at 525 nm, as you did for the other solutions. Record this value in your notebook.
Results and Analysis
Week 1
From the amounts of the reactants that you actually used, calculate the theoretical yield of alum (Help Me).
Week 2
Calculate the percent yield of alum from the theoretical yield you determined last week and the amount of alum that you actually obtained. Share your percent yield with your classmates.
Determine the percent water by weight in alum and the number of waters of hydration in the alum. Share these numbers with the rest of the class, as instructed. Perform a Q-test (Help Me) on the class data, and discard the discordant datum, if there is one. From the class data, calculate the average percent water by weight in alum, the standard deviation (Help Me) of the data and determine the confidence limits (Help Me) at 95% confidence. Based on the known formula for alum, determine the expected value of the percent water by weight in the sample. Calculate a percent error for the class average and for your result. Record all data and calculated results in your notebook. You may do the calculations in Excel, and if you do, you will need to paste copies of your output in your laboratory notebook.
Week 3
Calculate the concentration of the aluminum stock solution (the solution that you had after the second dilution) and the concentration of aluminum in each of the solutions that you prepared from the stock solution. Write these values in your table (Table 1, above) in the Results section of your notebook. In your calculations assume that the volumes of the flasks and pipets are as shown in Table 2.
| Volumetric | Volume (mL) |
|---|---|
| 1-mL Pipet | 1.00 |
| 2-mL Pipet | 2.00 |
| 3-mL Pipet | 3.00 |
| 5-mL Pipet | 5.00 |
| 25-mL Pipet | 25.00 |
| 50-mL Pipet | 50.00 |
| 100-mL Pipet | 100.00 |
Table 2. Nominal volumes of the volumetric glassware used in this exercise.
From the equation for the best-fit line for the absorbance at 525 nm as a function of [Al3+] determine the percent aluminum by weight in alum (Help Me) and share your results with the class. Perform a Q-test (Help Me) on the class data and then calculate the average percent Al by weight in alum, the standard deviation (Help Me) of the data and finally find the confidence limits (Help Me) at 95% confidence. Determine what the true percent Al by weight is for alum and then calculate a percent error for the class average and for your result. Record your calculations in your notebook, as you did for the Week 2 calculations, and include any spreadsheet output.
Conclusions
The first week of this exercise was a synthesis. Therefore, your Discussion of Conclusions section for this week should follow the synthesis outline. Note that you will not be able to discuss your results for Week 1 until after you have obtained the mass of your product and done the qualitative tests on it. It is advisable to reserve two or three pages in your notebook for the Week 1 Discussion of Conclusions and Error Analysiswhen you prepare for Week 2. One important question that you will need to address in your Discussion of Conclusions section for Week 1 is why is your percent yield of alum less 100% with specific references to what you did and observed.
Weeks 2 and 3 are both measurement exercises. In the Week 2 Discussion of Conclusions and Error Analysis you should include a brief discussion of the qualitative test results. In both Week 2 and Week 3 you gather evidence for the identity and purity of your alum. So, you must include a short discussion of whether your quantitative results support your purported synthesis of alum. Although a propagation of error analysis is possible, we won’t perform one here. However, you should be able to identify where your major sources of uncertainty are and qualitatively discuss how they affected your results.
Summary of Results
Week 1
Use Table 3 to report your results for Week 1.
| Mass of Al Used (g) | Theoretical Yield of Alum (g) | Mass of Alum Obtained (g) | % Yield of Alum |
|---|---|---|---|
Table 3. Summary table for the first week.
Week 2
Summarize your results for Week 2 using Tables 4 and 5. In the second column of Table 4, write either “positive”, or “negative”, as appropriate. Don’t forget to report the confidence interval on the class data in Table 5.
| Test for potassium: | |
| Test for sulfate: |
Table 4. Summary table for the qualitative tests.
| Initial Mass of Alum (g) | Mass of Anhydrous Alum (g) | % Water by Mass in Alum | % Error in % Water by Mass | Number of Waters of Hydration | |
|---|---|---|---|---|---|
| Our Results | |||||
| Class Results | ---------- | ---------- |
Table 5. Summary table for quantitative determination of water in alum.
Week 3
Table 6 should be used to summarize the results for the third week’s work. Remember to include the confidence interval on the class average % Al by mass in alum.
| Mass of Alum Used (g) | Slope of Calibration Curve (AU·M-1) | Intercept of Calibration Curve (AU) | Abosrbance of Alum Solution (AU) | % Al by Mass in Alum | % Error in % Al by Mass | |
|---|---|---|---|---|---|---|
| Our Results | ||||||
| Class Results | ----------- | ----------- | ----------- | ----------- |
Table 6. Summary table for Week 3.
- 1. Click here to download this file in PDF format. Note that hyperlinks are not active in the pdf version.
- 2. Smith, W. H.; Sager, E. E. and Siewers, I. J. Anal. Chem. 1949, 21, 1334-1338.
- 3. Marczenko, Z. Spectrophotometric Determination of Elements; Ellis Horwood Ltd.: Chichester, England, 1976, p. 116-117.

