Inorganic Qualitative Analysis1,2,3
Authors: B. K. Kramer* and J. M. McCormick
Last Update: January 15, 2013
Introduction
Qualitative analysis is the identification a sample’s component(s). Unlike a quantitativeanalysis, we are not concerned with the amount of a substance present in a sample but only with its identity. In this exercise we will focus on identifying the cations and anions that make up ionic compounds, both solid and in solution. Ideally there would be chemical tests that could be used to identify individual ions without interference by any other ions. Unfortunately, there are often complications. For example, the formation of a yellow precipitate upon addition of aqueous S2- confirms the presence of Cd2+ in a solution. The color of this compound, however, will be hidden if any Pb2+ or Cu2+ are present in solution since they will form a black precipitate with added S2-. In order to test for cadmium, then, any interfering ions must first be removed. This will be the case for most ions in a mixture: before their identities can be confirmed, they must be isolated from the remaining solution.
The separation of ions in solution can be accomplished by the addition of a precipitating agent that will selectively react with an ion in the solution and not with others that may be present. The solid that is produced can then be removed from the liquid by centrifugation and decanting. Because many ions may behave similarly, separation of individual ions from a complex mixture is not usually possible. Instead, a group of ions with similar reactivity may be separated by precipitation from a larger mixture. After they are isolated in a solid, they must be further separated and reacted to confirm each one’s identity.
There are several types of reactions that can be used to confirm the presence of ions in solution. The most common are precipitation and complexation. In a precipitation reaction, an ion in solution reacts with an added reagent to form a solid. Whether a solid will form from a given reaction can be predicted by the solubility product constant (Ksp) of the solid under the given conditions. Solubility product constants are the equilibrium constants for the dissolution of an “insoluble” ionic solid in water. A low Ksp implies that the compound does not dissolve to an appreciable degree in water. If the two ions are mixed in solution, a precipitate will tend to form. If steps have been taken to remove ions that form competing precipitates, the presence of a properly colored solid can be used to confirm the presence of a given ion. If several different precipitates remain, the conditions of the solution can be manipulated to selectively redissolve one or more of the solids. When the equilibria involved are well understood, selective precipitation can be a powerful tool in the identification of unknown ions.
Complexation can also be used to determine the presence of an ion in solution. In a complexation reaction, a cation (typically a metal) forms covalent bonds with one or more ligands (More Info). A ligand is a neutral or negatively charged species that donates electrons to the positively charged metal to form a coordinate covalent, or dative, bond. These complexes may either be neutral or charged, depending on the charge on the metal and on the ligand. When a complex forms it may not precipitate (charged complexes are often quite soluble in water, for example), and the formation of a complex is one way in which an insoluble metal ion can be forced to dissolve. Similarly, complex formation can also be used to separate a mixture of ions by keeping one or more in solution while others precipitate. If the complex formed between a metal ion and a specific ligand has a distinct color, complex formation can be used to demonstrate the presence of a specific metal ion by simply adding the ligand to the solution. They are useful in confirming the presence of a single ion after separation has been achieved. The tendency to form a complex can be determined by the formation constant (Kf) of the reaction. Formation constants are defined as the equilibrium constant for the reaction of the metal ion with the ligand(s) to form a complex. A large Kf implies a strong tendency for complex formation.
Qualitative analysis schemes have been performed by introductory chemistry students for many years. They are used to help students understand reactivity and to develop problem solving skills. There are several different approaches to these experiments. In one case, students are given a step-by-step procedure, often in the form of a flow chart, which they can use to isolate and identify unknown ions in solution. In another, students first analyze known solutions to determine how different anions will behave when reacted with various reagents. They then compile these results into their own flow chart that they apply to their unknowns. The experiments can be carried out on solutions containing mixtures of cations (same anion), mixtures of anions (same cation) or on salt mixtures.
The creation of the flow chart from scratch is very valuable but is also a very time consuming process. Your experiment will involve a modification of the flow chart procedure. The reactivity of the different ions with precipitating agents can be predicted based on the Ksp of the salt formed if the two were to react. You will use the Ksp‘s of several salts to determine the best way to cause their separation. You will then prepare a flowchart to separate and identify these components. You will test this flowchart in the lab. Ideally you would create a flowchart for the separation of the entire mixture. Because of time constraints, the flowcharts for the remaining species you need to separate will be given to you.
The overall experiment has three parts. In the first part you will analyze known mixtures of cations using your predetermined procedure and procedures that are given to you. This part is expected to take one to one and a half weeks. In the second part you will apply similar procedures to known mixtures of anions in solution. This part should take less than a full laboratory period. In the final week you will be given three unknown mixtures: a solution of three unknown cations, a solution of three unknown anions and a solid salt consisting of a single cation and a single anion. You will need to use the procedures you learned the previous weeks to identify the components of your unknowns.
The separation flow chart for the cations and anions encountered in this exercise are shown in Fig. 1 and Fig. 2, respectively. These flow charts show the steps required to separate and identify the cations and anions that you may find in your known and unknown mixtures. These charts have been prepared based on theoretical information about the ions and experimental observations. The flow charts can help you understand the order in which separations must take place in order to isolate ions that may behave similarly.
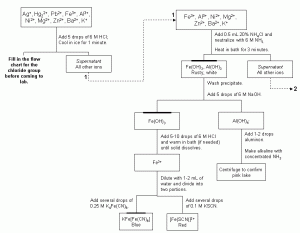
Figure 1. An example cation separation scheme for this exercise. See Fig. 3 for an explanation of the symbols used. You will need to prepare a flow chart for your instructor’s approval for the chloride group (Ag+, Hg22+, Pb2+) before starting this exercise. Click here to obtain these flow charts in PDF format suitable for printing.
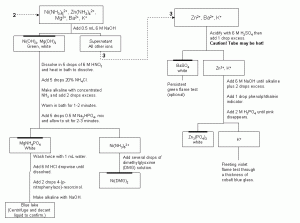
Figure 2. An example anion separation scheme. See Fig. 3 for an explanation of the symbols used. Click here to obtain this file in PDF format for printing.
Throughout the flow charts, reagent additions and other procedures are indicated along the connecting lines; these are explained in more detail below. The formula for each species, along with any identifying physical characteristics (such as color), is given in the box. The symbols and formalism used in the flow charts are given in Fig. 3.
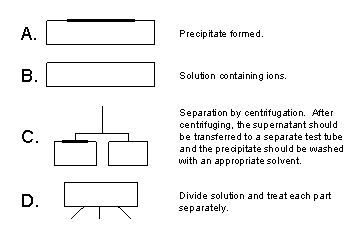
Figure 3. Key for the ion separation flow charts given in Fig. 1 and Fig. 2.
General Procedures
Since this analysis is qualitative and not quantitative, it is not necessary that exact amounts of reagent be added at each step, but it may be useful to know that there are approximately 20 drops in 1 mL. Each procedure should be performed on approximately 0.5 mL of a fresh sample of solution. IMPORTANT! Since some ions removed early in a given procedure may mask those determined later, it is essential that the entire chart is followed in order.
You will be using microcentrifuge tubes throughout this procedure. The tubes can hold either 1.5 or 2.0 mL (listed on the tube). If the volume of your solution exceeds that of the tube, separate the solution into two tubes and treat each one according to the flowchart.
Throughout this series of experiments, you will be expected to follow the directions that are presented in the flow charts included in this lab. The reactions shown in the charts are described in the accompanying text. There are several procedural steps that are indicated in the flow chart that are described here.
- Precipitation After the addition of a precipitating agent, it is important to mix the solution thoroughly by shaking or by stirring with a clean glass stir rod. Be sure not to add more of a precipitating agent than indicated in the procedure as this may cause undesired side reactions.
- Separation After the addition of a precipitating agent to a solution, a solid (precipitate) and liquid (supernatant) will result. These often must be isolated and treated separately. The most common method for separating a supernatant and precipitate is to centrifuge the mixture to cause the solid to compact at the bottom of the tube. The procedure for centrifuging will be demonstrated by your instructor. It is essential that the centrifuge is balanced with a tube containing the same volume of liquid as your sample to prevent it from “walking” off the table!! After centrifuging, the supernatant can be decanted by simply pouring from one test tube into another or by careful removal with a clean pipet.
- Washing When a supernatant is removed from a solid, it is almost certain that some of the liquid has been left behind. This liquid can be removed by the addition of a clean solvent (usually cold water, but indicated in the procedure if not) which is thoroughly mixed with the precipitate. After centrifuging and decanting, the solid is now ready for further reaction as dictated in the procedure. IMPORTANT! Improper separation and washing of precipitates is the most common source of error in this exercise. So, be sure that you learn how to do this properly using the known solutions.
- pH Adjustment Often it is necessary to adjust the pH of a solution until it is justalkaline or just acidic. This is usually accomplished by the dropwise addition of a strong acid or base. In order to make sure that the solution does not become too acidic or basic, the pH of the solution must be monitored. You will use universal indicator paper to determine the pH of your solutions. The proper method for finding the pH of a solution involves stirring the solution with a clean glass rod and then touching the tip of the rod to a piece of indicator paper. Do NOT place the indicator paper directly in your solution! You should check the pH of the solution after each addition of a drop of acid or base. If you are to acidify, stop the addition as soon as the paper registers a pH of just less than 7. If the solution is to be made basic, add base until the paper registers just more than 7. If you are to neutralize the solution, add the appropriate acid or base until the paper reads very close to 7.
- Heating Solutions There are two different heating methods that will be used in this exercise. If a solution needs to maintain a near boiling temperature for a few minutes, the tube should be placed in a boiling water bath. Microcentrifuge tubes can be heated by placing test tubes full of water in a water bath. After allowing the water to boil, place the microcentrifuge tube in the top of the test tube, making sure that the liquid contained is fully immersed in hot water. Make sure that the test tube is anchored so that it does not spill into the bath.
- If the solution needs to be heated directly, the solution should be transferred to a glass test tube which should be held in a test tube clamp facing away from you and your fellow students and passed back and forth through the flame of a Bunsen burner. Be careful not to let the solution bump and jump out of the test tube by keeping the flame near the surface of the solution rather than at the bottom. Stirring the solution with a glass rod may also help. The procedures will indicate which heating method is necessary for each step. DO NOT place a plastic microcentrifuge tube in or near a flame.
- Flame Tests In many cases, the color emitted when a cation is heated directly in a flame can help identify the element. If a procedure calls for a flame test, follow the directions below.
-
- 1) Clean a wire loop by first dipping it in 6 M HCl and then heating it long enough to drive off any contaminants from the surface.
- 2) Place the loop in the solution or solid to be tested, making sure a drop of liquid or crystal remains in the loop.
- 3) Place the wire in the flame and observe the color emitted. If instructed, view the flame through a piece of cobalt blue glass.
Throughout the experiment, you will use a flow chart (Fig. 1) to help you separate and identify the cations in your system. The first part of the chart has been left blank. Using the information in the paragraphs below, propose the steps to fill in the flow chart to isolate the chloride group (Ag+, Hg22+ and Pb2+) from a mixture, separate each ion from the others and confirm the presence of each ion. You will not be allowed to begin your experiment until your instructor has confirmed that your flow chart is prepared correctly. Your instructor will then give you a completed chart that includes the amounts of each solution to be added in each step.
Silver, mercury(I) and lead(II) are often called the “chloride” group because they form sparingly soluble to insoluble precipitates with chloride ions. All three solids are white. The first step in isolating these ions from a solution is to add HCl to form the chloride precipitates. Silver and mercury(I) chlorides are much less soluble (Ksp values of 1.8 x 10-10 and 1.3 x 10-18, respectively) than lead(II) chloride (Ksp of 1.6 x 10-5). If solid PbCl2 is heated in water to 100 oC for a few minutes, it will dissolve. The other two chlorides will not. Lead(II) in solution will form an insoluble white precipitate when allowed to react with sulfuric acid (Ksp = 6.3×10-7). The addition of ammonia to solid silver chloride causes the formation of a colorless silver-ammonia complex ([Ag(NH3)2]+, Kf = 1.7 x 107). The addition of nitric acid will cause the equilibrium to shift to free the silver which can then react with the chloride again. Mercury(I) chloride reacts with ammonia to form Hg (metallic liquid), HgNH2Cl (s, white), and Hg2O (s, black). The solid mixture will have an overall grayish color.
Before examining an unknown mixture it is helpful to observe the behavior of known ions in a mixture. You will separately analyze two known mixtures of cations using the procedures outlined in Fig. 1. Mixture A contains silver(I) (Ag+), mercury(I) (Hg22+), aluminum (Al3+), barium (Ba2+) and potassium (K+) ions. Mixture B contains lead(II) (Pb2+), iron(III) (Fe3+), nickel(II) (Ni2+), magnesium (Mg2+) and zinc (Zn2+) ions. There will also be solutions available that contain the individual ions that you will be analyzing. You can use these solutions to confirm the behavior of the ions in your mixture.
For each sample, you should record every step of the analysis and your observations as you proceed in a table similar to the one shown as Table 1. You should be as specific as possible when describing your observations of the known mixtures so that you can use those observations to identify your unknowns.
| Step Number | Prodcedure | Observations |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 |
Table 1. Sample data table for recording the results of each experimental step.
The first steps in the cation procedure are to identify and remove the chloride group as described above. After these have been isolated, the remaining cations will be separated based on their reactions with hydroxide. The reactions of hydroxide ions with cations are very interesting. By carefully controlling the pH of the solution, only certain metal hydroxides can be caused to precipitate from solution or form soluble complexes. After the chloride group ions are precipitated with hydrochloric acid, the solution will be acidic. An ammonia/ammonium buffer is then created in order to make it just neutral, which will shift the hydroxide concentration of the solution causing the precipitation of only the most highly insoluble hydroxides, Fe(OH)3 (Ksp = 1.6 x 10-39) and Al(OH)3 (Ksp = 3 x 10-34). It is important that the solution is not made overly basic as the additional hydroxide will cause the [Al(OH)4]– complex to form too soon. The remaining ions will either form soluble complex ions with the added ammonia or remain dissolved in solution. After separation from the supernatant, the aluminum hydroxide can be re-dissolved by increasing the concentration of hydroxide ions with the addition of sodium hydroxide. This addition will favor the formation of the complex, [Al(OH)4]– (Kf = 2.0 x 1033). However, the iron(III) hydroxide will not re-dissolve. Aluminum can be confirmed by adding aluminon, a dye, and then making the solution alkaline with concentrated ammonia. The presence of a pink lake (dyed precipitate) suspended in solution confirms the presence of aluminum. Make sure that it is not the solution itself that is pink by centrifuging. The presence of Fe3+can be confirmed in two ways. Iron forms a red complex with SCN– and a blue solid, KFe[Fe(CN)6], upon the addition of K4Fe(CN)6.
After the iron and aluminum are removed from the solution, the hydroxide concentration can be manipulated, again, to selectively precipitate two cations. An increase in the concentration of hydroxide ions with the direct addition of aqueous sodium hydroxide will cause the precipitation of nickel hydroxide (Ksp = 6 x 10-16) and magnesium hydroxide (Ksp = 6 x 10-10). The other cations will remain in solution; zinc as the hydroxide complex [Zn(OH)4]– and Ba2+ and K+ as the solvated ions. Like most hydroxides, magnesium and nickel hydroxide can be dissolved in a solution that is acidified and warmed. After re-establishing the ammonia buffer, the addition of Na2HPO4 will cause the magnesium to slowly precipitate as MgNH4PO4. Nickel will remain in solution in the form of a nickel ammonia complex. The magnesium can be redissolved in hydrochloric acid. The magnesium will form a blue lake in an alkaline solution containing the organic compound 4-(p-nitrophenylazo)-resorcinol. Dissolved nickel ions will form a deep pink precipitate upon the addition of another organic compound, dimethylglyoxime.
The only ions remaining in solution after the hydroxide concentration is raised are zinc, barium and potassium. Barium forms an insoluble precipitate with sulfate ions (Ksp = 1.1 x 10-10). The barium can be further confirmed by the presence of a persistent green flame test. Zinc can be precipitated by the addition of phosphoric acid, H3PO4 (Ksp for Zn3(PO4)2 is 5 x 10-36).
At this point, the only unknown ion remaining in solution will be potassium. Potassium forms very few insoluble precipitates. The simplest way to identify it is by a flame test after other ions are removed. The flame will turn a fleeting violet color when exposed to potassium ions. Because this color may be masked by the orange flame of sodium ions, the flame should be viewed through a thickness of cobalt blue glass.
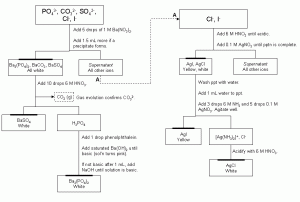
The strategy for the analysis of anions is similar to that for cations; known reagents are added to a solution to selectively precipitate dissolved anions. In this case, the precipitating reagents will be cations that form insoluble salts with the dissolved anions. You will perform an analysis to identify chloride (Cl–), iodide (I–), carbonate (CO32-), sulfate (SO42-) and phosphate (PO43-). The flow chart for the separation and identification of these anions is shown in Fig. 2. You will be given only one known solution to analyze in this section. This mixture will contain all five anions.
The first step in this procedure is the addition of barium nitrate to cause the precipitation of BaCO3 (Ksp = 5.0 x 10-9), BaSO4 (Ksp = 1.1 x 10-10) and Ba3(PO4)2 (Ksp = 6 x 10-39). The addition of a strong acid (nitric, HNO3) to these precipitates will adjust the solubility of the ions by taking advantage of their basic nature. The reaction of CO32- and the acid will cause the evolution of carbon dioxide gas. Because of the limited number of anions possible in your procedure, the presence of this gas is a confirmation for the carbonate ion. The nitric acid will also cause the dissolution of barium phosphate. When the supernatant containing only barium phosphate is decanted and made basic with Ba(OH)2the precipitate will reappear. Unlike the other two precipitates, barium sulfate will not redissolve when nitric acid is added. The presence of a white solid after the acidification of the barium precipitates is a confirmation of the sulfate ion.
The supernatant found after the addition of barium in the previous step will contain the other anions in this procedure, I– and Cl–, neither of which form insoluble salts with barium. (This procedure could be performed on a fresh sample of the analyte, as well.) They do, however, form insoluble salts with silver ions (Ksp(AgCl) = 1.6 x 10-10, Ksp(AgI) = 1.5 x 10-16). The solubility of these ions can be further decreased by acidifying the solution with nitric acid. The precipitate should be washed to remove any other ions and then stirred in clean distilled water. When aqueous ammonia and additional silver nitrate are added to the mixture, the silver chloride will redissolve to form the silver ammonia complex used in the detection of silver ions. The yellow silver iodide will not redissolve. If the supernatant containing the complex is acidified with nitric acid, the silver chloride will reprecipitate, confirming the presence of chloride ions.
You will be given three unknowns to analyze. The first will be a solution containing three cations. The second will be a solution containing three anions. The third will be a solid binary salt. As soon as you receive your unknowns, record your unknown numbers. You must include your unknown numbers in your reports to receive credit. Use the procedures you learned in the first two weeks to determine the compositions of your three unknowns.
When analyzing unknown mixtures, you should keep a few things in mind. Remember that you must have a confirmatory test for each cation you believe is present. Even if you are told the number of ions present in your mixture, you should not stop after finding that number of ions. It is possible that you have made a mistake or have a false positive. Complete the entire flow chart to make sure that no other species appear. If another is identified, you should repeat the procedure on a fresh sample of analyte. You should have plenty of your solution to repeat the entire analysis several times. There may be penalties if you ask for extra, however, so be careful when using your unknown.
If you are ever uncertain as to whether a test is positive for a given ion, you can repeat the test on the standard solutions provided to confirm the behavior of that ion. After you have identified your unknown mixtures, you may want to create a mixture containing the ions you believe are present in your solution. If you have time, you can test this solution and compare the results to your unknown mixture.
When you are analyzing for both cations and anions in a single unknown, it is important to recognize that the analyses must be performed separately. For example, the first step for a cation analysis is the addition of hydrochloric acid. A test on a solution after HCl is added would, obviously, be positive for chloride ions!
The various analytical procedures you will do must be performed on ions dissolved in a solution. The first step in your solid unknown analysis will be to dissolve the sample. Your sample may be insoluble or sparingly soluble in distilled water! The fact that your unknown is insoluble in water may give you an idea as to its identity. Try dissolving a small sample of the solid first in water, then nitric acid or another aqueous solvent until you find a solvent in which your unknown is completely soluble. You should then use this solvent to prepare a mixture that is 1-3% of your unknown by weight. Try to avoid using a solvent that contains any of the possible unknown ions!
Results and Analysis
Report to your instructor the identity of the ions in each of your unknowns. Your instructormay allow you to repeat the identification of an incorrect result, so you should submit your decision as soon as possible after you have confirmed your unknowns’ identities. In the Results section of your lab notebook you should include the balanced net ionic equations of each reaction in the flow charts. You should attempt to determine these reactions and may be able to find some of them in your textbook or a reference text. Use equilibrium constants for the reactions to explain why one ion precipitated or formed a complex while others remained solvated. Some of the equilibrium constants you need are included in the procedures.
Conclusions
For this experiment you do not need to write any conclusions until you have finished the analysis of your unknowns. Use the outline for reporting on physical phenomena from the Laboratory Notebook web page as a starting point for your discussion.
Summary of Results
Use Table 2 to report the analysis of your unknowns.
| Unknown Number | Ions Found |
|---|---|
| 1 |
Table 2. Summary table for reporting the identity of the unknowns.
- 1. Click here to download this file in PDF format. Note that hyperlinks are not active in the pdf version.
- 2. Wismer, Robert K. Qualitative Analysis with Ionic Equilibrium; Macmillan Publishing Company: New York, 1991.
- 3. Zumdahl, S. S. Chemical Principles, 4th Ed.; Houghton-Mifflin: New York, 2002, chapter 8.