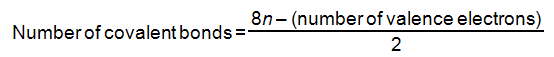How to Draw Lewis Structures
Last Update: February 4, 2011
The following is a general procedure for drawing Lewis structures. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. It is this similarity that allows us to understand the chemistry of complex molecules (especially organic molecules).
Remember that a Lewis dot structure is an approximation of the actual arrangement of electrons in a molecule or polyatomic ion, much in the same way a cartoon of a cat is an approximation of the actual animal. Our cartoon picture of a molecule is limited, but can give us a rough idea the bonds that hold a polyatomic chemical species together, which is why it is useful.
1. Determine type of compound.
Remember that Lewis dot structures
- only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements,
- can not predict the structure of ionic compounds,
- they are not useful for compounds (molecules or polyatomic ions) involving transition metal ions except those which have a d0 or d10 electronic configuration, for which they do work.
2. Identify the arrangement of the atoms.
- Identify the central atom.
-
- Usually this is the least electronegative atom (example: in CF4, C is the central atom).
-
- In simple compounds the central atom is the element that appears only once in the formula (example: H2O, there are two H and only one O, so O is the central atom, even though it is the most electronegative atom).
- H and F are almost always terminal. The primary exception to this rule is where H is bonded to more than one other atom. Examples of this are hydrogen bonding, and a family of compounds called boranes (e. g., B2H6). In these cases hydrogen is said “to bridge” the other atoms or is said to be “bridging”. Compounds of this type are rarely encountered in introductory chemistry, but you should be aware of their existence because they are not uncommon.
- All other halogens can be either the central or a terminal atom.
-
- The easiest way to tell which is which is to go by electronegativity or by the number of atoms.
-
- The terminal atoms when a halogen is in the center are usually O or another halogen.
-
- All halogens, except F, can form bridges, just like hydrogen. Like the other bridged structures discussed above, bridging halogens are not often encountered in introductory chemistry classes, but you should know that it is possible. An example of bridging halogens occurs in aluminum bromide (Al2Br6) where two Br bridge the Al and the other four Br are bound terminally.
- Unless it is clearly the central atom, oxygen is a problem, because it can be a terminal atom (as in CO2) or a central atom (as in H2O). The best course of action is to leave it until last. Some of the most vexing examples are organic compounds where oxygen is bound to carbon. Examples of these are:
-
- Ethers (O is the central atom between two C),
-
- Alcohols (O is the central atom between C and H),
-
- Aldehydes, ketones and amides (O is attached terminally to one C, in an amide the C is also bonded to an N),
-
- Esters and carboxylic acids (two O are attached to one C, one O is terminal and the other is attached to another atom).
- When all else fails, go with the arrangement that gives the most symmetric structure.
3. Determine the total number of valence electrons.
- Add up the number of valence electrons from all atoms.
- For anions add a number of electrons equal to the negative charge.
- For cations subtract a number of electrons equal to the positive charge.
4. Place one electron pair between each pair of adjacent atoms (as determined from the framework found in step 2) to form a single bond.
5. Place electron pairs around each terminal atom (except H) to complete octets.
6a. If not all of the electrons have been placed and all terminal atoms have complete octets, place the remaining electrons pairs on the central atom.
- Be sure to recognize the elements that violate the octet rule by having more than 8 electrons (elements of the third and higher periods).
- If you have electrons left over and the central atom can not violate the octet rule, then there is a mistake somewhere. Common errors include: wrong number of valence electrons, wrong framework (central atom is incorrect) and simple math/counting errors.
6b. If the central atom doesn�t have a complete octet, move a lone pair from a terminal atom to a position between the terminal and central atoms. This gives a double bond. Repeat as needed to form other double bonds using other terminal atoms, or a triple bond if no other terminal atoms are available.
- Hydrogen never forms a double or triple bond.
- Carbon, oxygen, nitrogen and sulfur are the most commonly encountered atoms that will form multiple bonds. So, look for these first when you need to form multiple bonds.
- Halogens seldom form multiple bonds when they are not the central atom.
-
- The primary exception is where the central atom can’t complete its octet (e. g., BF3).
-
- Use the Electroneutrality Principle (see point 8, below) to guide you. If it violates the Electroneutrality Principle, then forming the extra bonds is probably not a good idea.
- Halogens that are central atoms can form multiple bonds with the atoms around it because they can expand their octet (see points 7 and 8).
Langmuir’s Rule can also help you decide whether you should form multiple bonds. Langmuir’s Rule states that for a molecule or polyatomic ion where n atoms complete their octets, the number of covalent bonds is given by the equation shown below. Note that this rule does not work for compounds containing hydrogens or that have atoms that have expanded octets.
|
|
7. Consider resonance.
Resonance is an attempt to show bonds that do not involve a pair of electrons being shared by two atoms. The keys to spotting resonance are:
- atoms that have a single bond on one side and double or triple bond on the other,
- atoms with a double bond adjacent to an atom with lone pairs,
- atoms that can exceed the octet bonded to an atom with lone pairs.
- Langmuir’s Rule is sometimes useful in spotting resonance, too.
8. Calculate formal charges and use the Electroneutrality Principle to determine which Lewis dot structure is the best, or which resonance structure makes the largest contribution to the resonance hybrid.
The Electroneutrality Principle states:
- electrons are distributed so that charges on atoms are as close to zero as possible,
- best structures are those that minimize formal charge and don’t separate formal charge,
- if charges must be placed on an atom, a negative charge should sit on the most electronegative atom or a positive charge should sit on the least electronegative atom,
- structures with more covalent bonds are better Lewis dot structures (as long the above rules are also satisfied).