Typical Vibrational Frequencies of Inorganic Species1
Last Update: July 26, 2005
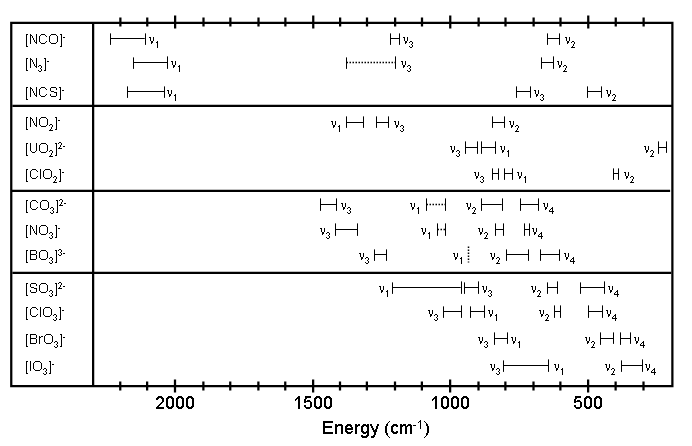
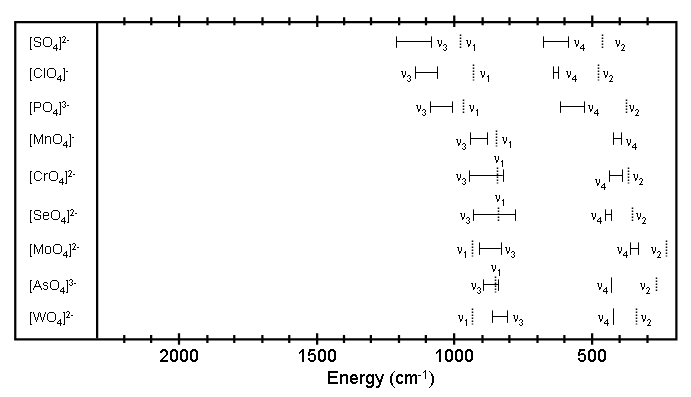
Figure 1.2 Observed frequency ranges for polyatomic ions. See reference 2 for a description of the vibrations associated with each frequency. Raman active bands are shown as dashed lines.
| BF4- | PF6- | HCO3- | NO2- | NH4+ |
|---|---|---|---|---|
| 1128 | 913 | 1295 | 1328-1375 | 3125 |
| 1107 | 830 | 1035 | 1261-1270 | 1401 |
| 1088 | 557 | 1000 | ||
| 1077 | 838 | |||
| 1063 | ||||
| 1038 |
Table 1. Approximate IR frequencies (in cm-1) of other common polyatomic ions not shown in Figure 1.3
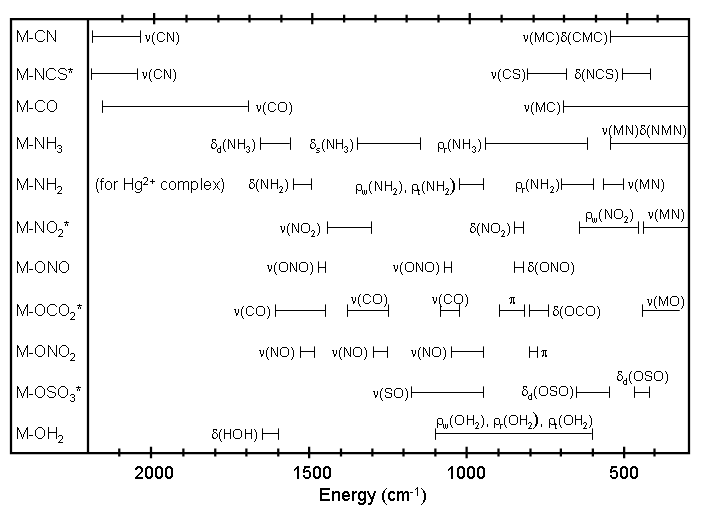 Figure 2.2 Metal-ligand vibrations. See reference 1 for a complete discussion of the motions associated with each vibration. The ranges for species marked with an “*” include both bridged and non-bridged compounds. Whether a particular vibration is IR or Raman active depends on the particular geometry about the metal center.2, 4, 5, 6
Figure 2.2 Metal-ligand vibrations. See reference 1 for a complete discussion of the motions associated with each vibration. The ranges for species marked with an “*” include both bridged and non-bridged compounds. Whether a particular vibration is IR or Raman active depends on the particular geometry about the metal center.2, 4, 5, 6
References
1. Click here to download this file in PDF format (link not yet active).
2. Nakamoto, K. Infrared Spectra of Inorganic Coordination Compounds, 2nd Ed.Wiley-Interscience: New York, 1970.
3. Dolphin, D. and Wick, A. Tabulation of Infrared Spectral Data Wiley-Interscience: New York, 1977.
4. Drago, R. S. Physical Methods in Chemistry W. B. Saunders: Philadelphia, 1977.
5. Ebsworth, E. A. V.; Rankin, D. W. H. and Cradock, S. Structural Methods in Inorganic Chemistry, 2nd Ed. Blackwell Scientific Publications: Boston, 1991.
6. Wilson, Jr., E. B.; Decius, J. C. and Cross, P. C. Molecular Vibrations: The Theory of Infrared and Raman Vibrational Spectra Dover: New York, 1955.
