Kinetics and Activation Energy of a Diels-Alder Reaction1
Adapted from reference 1 by J. M. McCormick
Last Update: February 5, 2014
Introduction
The highly-colored compound 2, 5-dimethyl-3, 4-diphenylcyclopentadienone (I) undergoes a reversible Diels-Alder reaction2,3 to give the colorless dimer (II), as shown in Scheme 1. In this exercise you will determine this reaction’s rate law and activation energy and explore whether the activation energy is solvent dependent.
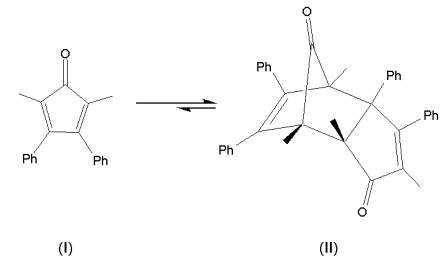
Scheme 1. The Diels-Alder reaction of 2, 5-dimethyl-3, 4-diphenylcyclopentadienone (I).
Experimental
Prepare the spectrometer to obtain the absorbance at 460 nm (e460 = 225. M-1·cm-1) as a function of time. You will not need to set a delay time for the spectrometer to wait before it acquires data as we will arbitrarily set the t = 0 point. Data should be obtained in approximately 2 min intervals for about 20 min. There is no need to obtain Ainfinity because the product does not absorb at 460 nm.
Dissolve approximately 15 mg of (II) in 10 mL of toluene, xylene or mesitylene. Heat the solution in a hot water bath until the solution becomes highly colored. Cool the solution on an ice bath until it is cool to the touch. Transfer the solution to a cuvette and place in the water jacketed cell holder that is already equilibrated at the desired temperature. Wait 5 min for the temperature to equilibrate and start data acquisition (this will be t = 0). Make three measurements at 25.0 °C and then once each at least three other temperatures, as time permits (10 °C, 15 °C, 30 °C and 40 °C may be good choices). You do not need to prepare a fresh sample each kinetics run; simply reuse the same solution by reheating it in the hot water bath. Repeat the temperature dependence in solvents of your choice (these must be non-polar organic solvents that do not absorb at 460 nm).
Results
Convert the raw absorbance as a function of time to concentration of (I) as a function of time. Use the integrated rate laws to determine the rate law and the rate constant for this reaction. Once the rate constant and rate law are known, use the Arrhenius equation to find the activation energy for this reaction. Determine the uncertainty in the measured rate constants and the activation energy.
Conclusions
Discuss your results given the known mechanism of the Diels-Alder reaction.
1. Weiss, H. M. and Touchette, K. J. Chem. Educ. 1990, 67, 707-709. Click here to obtain this paper in PDF format (Truman addresses only).
2. Solomons, T. W. G. Organic Chemistry, 4th Ed.; Wiley: New York, 1988, p. 470-475.
3. Sykes, P. A Guidebook to Mechanism in Organic Chemistry, 5th Ed.; Longman: New York, 1981, p. 328-344.
