The Laboratory Report1,2
Authors: M. C. Nagan and J. M. McCormick
Quick Links
| Page Setup and Formatting | Title | Conclusions |
| Style | Abstract | References |
| Organization | Introduction | Tables |
| Template | Experimental | Schemes |
| Results | Figures | |
| Discussion | Example Lab Report |
Introduction
The research paper is the primary means of communication in science. The research paper presents the results of the experiment and interpretation of the data, describes the rationale and design of the experiment, provides a context for the results in terms of previous findings and assesses the overall success of the experiment(s). Scientists working in industrial laboratories do not write as many journal articles as their colleagues in academia, but they routinely write progress reports, which take the same form as a journal article. So no matter what your career goals are, it is important that you become familiar with this style of writing.
There are set rules for preparing a journal article (or a laboratory report). The style requirements vary only slightly from journal to journal, but there are far more similarities than differences in the scientific writing style. If you are writing an article for publication in a particular journal (or preparing a laboratory report in the style of a particular journal) you should consult the Instructions to Authors section of the journal’s website (this information is also included in the journal’s first issue of each year).
There are several style guides3,4 and articles5 to help scientists and students prepare their manuscripts. The most useful of these to chemists is the American Chemical Society’s (ACS) ACS Style Guide, which may be found in the Truman library or may be purchased from the ACS web site. Because of the variation in journal styles, and the requirements for a specific course, your instructor will inform you of specific style requirements for his or her class. This guide is based on the Journal of the American Chemical Society style,6 and is meant to provide a good starting point for writing a laboratory report. It is not meant to be the definitive style guide; you must adjust your style to your audience and the journal in which your results will be published.
Although we shouldn’t, all of us are swayed by first impressions. How your paper appears to the journal editor or reviewer is their first impression of your science, and it will color their impression of your results, if you let it. Nothing is worse than a sloppily prepared paper with no page numbers, a font that can’t be read or which is full of grammatical errors. Remember that everyone will assume that if you did not take the time to write your paper carefully, you did not take the time to do your science carefully.
The following are some general editorial guidelines to follow that will leave a good first impression with your readers.
- 1) Double-space your paper throughout (including figure captions and tables, too).
- 2) Use a reasonably sized font such as 12 point Times.
- 3) For figures, you may choose to use a sans-serif font for better graphics quality such as Arial or Helvetica.
- 4) Use at least 1” margins on all sides.
- 5) Number the pages. Place the page numbers in the top, right-hand corner, or centered on the bottom of the page. Either style is acceptable and whichever one you choose remain consistent in your numbering scheme throughout the paper.
- 6) Do not start sentences with symbols or numbers; rather, spell out the full name of the symbol if it is used at the beginning of the sentence. For example, write “Alpha-lactalbumin” instead of “α-Lactalbumin” when beginning a sentence. Also spell out symbols or numbers in a title, except when part of a chemical name (e.g. 2-hexanol).
- 7) Spell check the document thoroughly. Have someone, who will give you an honest and complete critique of your paper, read the paper. Revise, revise, revise!
Uniformity of style is the key to scientific communication. The journal editors, the referees who review a manuscript, and the journal readers who are interested in the results presented in a paper all expect certain things to be present in a manuscript and that they are in a certain order. Just like the sloppy-looking paper, a paper that does not adhere to the expected style reflects poorly on the author, no matter how good the science is.
- 1) The paper should be written in a third person, passive voice. Occasionally, but rarely, it is appropriate to use “we” when describing the intention of the authors. It generally depends upon the intended subject of the sentence. Consider the two sentences below:
- a) Calcium solid (5 g) was poured into a beaker.
- b) We poured calcium solid (5 g) into a beaker.
- In the first sentence (a), which is passive, the subject is the calcium solid. In the second sentence, the subject is the experimenters. In scientific articles, the subject is most often the science and not the experimenters.
- 2) Use the past tense in general (e. g., what was or has been done). However, use present tense when describing properties of molecules or organisms because they still have these properties.
- 3) Unless directed otherwise, assume the reader of your laboratory report is your peer, the average chemistry student, not the chemistry professor. Therefore, everything should be explained as if the reader knows some chemistry, but is not an expert in the subject of the paper. By no means does the reader know what you are doing, or why you are conducting your experiment. Think about what you would want to know about the subject if you were the reader.
- 4) Avoid repetition in language. Try not to start each sentence with the same construction and words.
- 5) Do not use quotes. Unlike humanities or literature papers, quotations are rarely found in scientific articles. However, it is appropriate to paraphrase other authors.
- 6) Explain technical terms.
-
- Example
-
- “Hemoglobin has a Hill constant, a value that describes the degree of cooperative ligand binding, of 2.8.”
- 7) Define abbreviations.
-
- Example
-
- “The official colors of Truman State University (TSU) are purple and white.”
- 8) Place a space between a number and a unit.
-
- Example
-
- “Sephadex (10 g) was combined with deionized H2O (100 mL) at 25 °C.”
- 9) Do not start a sentence with a number or “Figure 1” or “Table 1”, etc..
-
- Correct: Milk samples (50 μL) were analyzed by high performance liquid chromatography under three different buffer conditions (Figure 1).
-
- Incorrect: Figure 1 shows the high performance liquid chromatography chromatograms for the sample run under three different buffer conditions.
-
- Incorrect: 50 μL of milk was analyzed by high performance liquid chromatography using three different buffer conditions.
- 10) There are three ways to refer to a paper in the text.
- For example, the citation of the work authored by Jackson, A. K.; Wilson, R. S.; Houk, K. L.*, could appear in the text in any of the following ways. (Note that et al. is an abbreviation for et alia and that it is italicized because it is not English.7)
-
- a) Jackson et al.
-
- b) Jackson and coworkers
-
- c) Houk and coworkers
In the last example we assumed that the author whose name is starred is the principle investigator on the project, and gave them more credit for the work. Note that it is an American convention to list the principle investigators last, while many European and Japanese journals place them first.
- Often there are two principle investigators, and in this case both should be mentioned. For example, the work by Jackson, A. K.; Wilson, R. S.*; Houk, K. L.* should be referred to, in the format given in example (c) above, as “Wilson, Houk and coworkers”. If there are more than two principle investigators, it is best to use either of the formats given in example (a) or (b), or to use some other wording to avoid this construction entirely.
Sections
Sections should appear in your paper in the order described below. All sections but the title have the section explicitly labeled, usually in bold letters to differentiate it from the rest of the text, and left aligned on the page. A blank line should appear after the last word of the section to separate the various sections, but a line should not be placed after the section title.
- 1) Title/Title page
- 2) Abstract
- 3) Introduction
- 4) Experimental (Materials and Methods in some journals)
- 5) Results
- 6) Discussion
- 7) Conclusions
- 8) Acknowledgements
- 9) References
- 10) Tables
- 11) Schemes
- 12) Figure Legends
- 13) Figures
- 14) Supporting Information
Please note that you should not physically assemble your paper in this order. Instead, it is suggested that you compose: a) Materials and Methods, b) Figures, Figure Legends and Tables, c) Results, d) Discussion, e) Conclusions, f) Introduction and Schemes, g) Abstract, and h) Title. Then put all the sections together in the final paper in the order outlined above.
A template is available to help you organize your report. Click here to learn more about it.
Subsections
It may be helpful to organize sections further into subsections. These subsections should have their own titles that are italicized and followed by a period.
Description of Paper Components
A title reflects the emphasis and contents of the paper. It tells the reader the paper’s topic and it also entices the reader to continue reading further. Therefore, it is not uncommon for the title to reveal the results or major conclusions of the experiment. Examples are given below. The title should be on its own page (the title page), left-aligned at the top of the page, in bold letters. Note that in some journals the title’s font size is 2 points larger than the text (i. e., 14-point, if the rest of the paper is in a standard 12-point font). However, this is not standardized and you should check with your instructor for which format he/she wants you to follow.
The title must be brief (2 lines maximum) and grammatically correct. Under the title, write your name and your professional address in italics (Department of Chemistry, Truman State University, 100 East Normal, Kirksville, MO 63501).
- Example Titles
-
- 1) Determination of the Differential Fluidity of Water and Benzene by Viscosity Measurements
-
- 2) Purification of Alpha-Lactalbumin from Bovine Skim Milk by Immobilized Metal Ion Affinity Chromatography
-
- 3) Synthesis and Characterization of Potassium Tris(oxalato)ferrate(III)
-
- 4) Ionic Composition of Drinking Water Influenced by Pipe Materials: An Atomic Absorption Spectroscopic Analysis
The abstract is a one-paragraph summary of the paper that is written in the present tense. As the abstract is the only part of the paper that is entered into article databases, it should be able to stand alone, separate from the paper. The first one to three sentences of the abstract should briefly introduce the reader to the problem studied. Next, the scientific approach, major results and primary significance of the findings should be presented. The abstract is generally 150-200 words (less for shorter papers). This section is normally written after the body of the paper. Because the abstract is separate from the paper, all abbreviations should be written out, or defined, and any references should be written out in full. An example of how a reference might appear in an abstract is
- Inhaled fumes from permanent markers have been shown to cause brain damage (Johnson, A. J. Permanent markers and the brain. J. Am. Brain. Res. 2004, 18, 215–218).
Note that in some journals that inclusion of the title in a reference is not required (vide infra).
The introduction should present the scientific problem at hand to the reader. Explain to the reader why the experiment was conducted, how it was designed and perhaps, if appropriate, what was found. Literature that is relevant should be incorporated and will help the reader understand the context of your study. A good rule of thumb is to start at the most general topic and progressively move towards the specific. Here is a general outline for an introduction:
- I. Broad significance of the topic to the chemistry discipline and society in general
- II. Introduction to the topic within chemistry
- III. Description of the specific problem
- IV. General goals and significance of the experiment or research topic
In this section, consider including figures, schemes and equations that complement the text.
While this is similar to the information that you should have written your notebook, the introduction to a paper is different than the background that you included for an experiment (or experiments) in your notebook. Remember that you are trying to reach a larger, more general audience with your paper, and the introduction must be structured to draw the reader in and help them focus on your important results.
The experimental section of your paper should be a logical, coherent recount of the experiment(s) conducted. This section should be complete enough for a trained scientist to pick up your report and replicate your experiment. The experimental section in a laboratory report is more concise than the corresponding section in the laboratory notebook. It should not be a step-by-step procedure of the activities carried out during the laboratory period.
The first paragraph of the experimental section contains information on key chemicals used in the procedure. When the chemicals are used as received, there will usually be a statement to that effect and further details are not usually necessary. You will list the chemical supplier’s name and the substance’s purity will be noted in cases where the chemical is hard to find, it is of a special purity or if there is only one supplier. Do not list lot numbers. If a starting material was synthesized according to a literature procedure, then state this in the opening paragraph and reference the procedure. If purification or drying of the compounds is required, it is described here, also.
The first paragraph often will also list the instruments used to characterize the newly synthesized substances. All instruments and equipment should be specified including the model number of the instrument and the name of the manufacturer (serial numbers are not included). When a spectroscopic or physical method is the focus of the report, it will be described in its own subsection. You are not required to write the experimental in this fashion.
For common techniques, laboratory textbooks should be referenced. However, if a previously published procedure was modified, then this is stated and only the modifications performed are included. If the procedure is your own, then outline the procedure with the main points, including details that are critical to replicating the experiment. These might include the type and size of your HPLC column, the buffer or the concentrations of chemicals.
When the syntheses of substances are reported, the synthetic procedure used to make each substance is described in its own separate paragraph. The paragraph begins with the name of substance, or its abbreviation (if the abbreviation was defined earlier in the paper), in bold face. If numbers are assigned to the compounds, these are also included (in parentheses). Often the synthesis will be written out, even when a literature procedure was followed. The mass and percent yields must be reported. Some of the new compound’s characteristics are included at the end of the paragraph describing its synthesis. These include: melting point range (and literature value, if known), elemental analysis (both calculated and found), selected peaks from the mass spectrum (with assignments), selected IR peaks (also with assignments), and any NMR peaks with their chemical shift, multiplicity and integration (you will often find the observed coupling quoted and the assignment of the peaks). The following is an example of how to report a compound’s synthesis.
- Tris-(2-pyridylmethyl)amine: To a stirred solution containing 10.11 g 2-pyridylmethyl chloride hydrochloride and 3.20 ml 2-pyridylmethyl amine in 20 ml H2O was added in a slow, drop-wise manner (~1 drop every 25 sec) a solution containing 5.03 g NaOH in 12 ml H2O so that all of the solution was added in about 1.5 hr. Upon complete addition of the NaOH, the reaction mixture was heated on a heating mantle to 70 ºC for 20 min. The cooled reaction mixture was then extracted four times with 50 ml CH2Cl2. The combined extracts were dried over Na2SO4 and the CH2Cl2 was removed using a rotary evaporator. The resulting red oil solidified upon standing. The red solid was then dissolved in a minimum of hot hexane. The yellow solution was decanted from a red oil which did not dissolve and filtered hot. Upon cooling the product crystallizes in large needles, which were recovered by filtration and air-dried. Recrystallization from hexane gave 2.08 g of the product (23% yield). The melting point of product is 85 ºC, sharp (literature 87 – 89 ºC).ref 1H NMR (CDCl3, ppm): 3.89 (s, 6 H, methylene), 7.14 (m, J = 1.3, 6.1 Hz, 3 H, pyridyl), 7.58 (d, J = 7.8 Hz, 3 H, pyridyl), 7.63 (m, J = 1.8, 7.6 Hz, 3 H, pyridyl), 8.15 (m, J = 0.9, 4.9 Hz, 6 H, pyridyl). 13C NMR (CDCl3, ppm): 60.13 (methylene), 122.01, 122.97, 136.48, 149.06, 159.25 (pyridyl).
The experimental section has two quirky wrinkles on the general scientific style. These are:
- 1) when citing previously published procedures, authors’ names are generally not included,
-
- Correct “Purification of the bovine brain isolate was performed according to previously published procedures.ref“
-
- Incorrect “The previously published procedure of Jackson et al.ref was followed with modifications outlined below.”
- 2) when citing the use of a kit, pre-packaged-assay or other commercial equipment with directions, include just the company’s name in parentheses; it should not be a full reference.
-
- Example
-
-
- “The Bradford assay (Sigma) was carried out to determine the total protein concentration of the five protein isolates.
-
In the Results section, the results are presented and summarized in a reader-friendly form. Raw data are not presented here. For instance, it is appropriate to include the average calculated concentration of a solution but not the original absorbance values that were collected from the spectrophotometer; that information is best left in your laboratory notebook.
Graphs and tables often make the data easier to interpret and more understandable (click here to review graph preparation). A graph is presented in the paper as a figure. In general, a graph or table is an appropriate representation of the data when more than 2 or 3 numbers are presented. Data that are presented in the form of a graph or table should be referred to but should not be repeated verbatim in the text as this defeats the purpose of a graph. More information on figures and tables is presented later.
The Results section also reports comparable literature values for the properties obtained and/or calculated in the paper. Observation of trends in the numerical data is acceptable. However, interpretation of the trend should be saved for the Discussion section.
Remember, do not simply report your numerical results. The Results section must have a narrative that describes your results. This narrative can include a description of the data (such as spectra or data in graphs), what problems were encountered during data acquisition (and how they were resolved, or not) and a general description of how the raw data were processed to give the final results (not a step-by-step description of everything you did). The reader wants to know what you did, how you did it, what problems you encountered and finally what your results were. Each of these topics must be addressed in the Results section in a way that is clear, yet concise.
This is the section where the results are interpreted. This section of the paper is analogous to a debate. You need to present your data, convince the reader of your data’s reliability and present evidence for your convictions. First, evaluate your data. Do you have good, mediocre, terrible, or un-interpretable data? Evaluate your results by comparing to literature values or other precedents. Explain what results should have been obtained and whether you obtained these expected values. Note that even if expected results were not obtained, you did not fail. Unexpected results are often the most interesting. Perhaps your hypothesis was not correct. Why is this? What new hypothesis do your data suggest? If you feel that your results are not reliable, you need to explain why. Use statistical analysis or chemical principles to support your claims. Was there a systematic error? Is the error due to the limitations of your apparatus? Does your data look the same to within a standard deviation? Evaluate the statistical significance of your data (click here to review the statistical treatment of data). After validating your data, you should interpret your results; state what you believe your results mean. How do your results help us understand the scientific problem? What do your results mean in the context of the bigger picture of chemistry, or of science? How do your results relate to the concepts outlined in the introduction? Do not assume that your experiment failed or was successful. You need to prove to the reader, with logical arguments and supporting evidence, the value of your study.
The conclusions that you wrote in your laboratory notebook are a good starting point from which to organize your thoughts. Your paper’s discussion section is structured very similarly to the conclusions section in your notebook, and it might be good idea to review that now (click here to review the structure of the conclusions in the laboratory notebook).
The Conclusions section is typically a one-paragraph summary of your laboratory report. Here you summarize the goal(s) of your experiment, state whether you reached that goal, and describe briefly the implications of your study. Note that in some chemistry sub-disciplines it is acceptable to combine the Discussion and Conclusions sections. Consult your course syllabus or check with your instructor on the specific format to be used in your class.
Acknowledgements
The Acknowledgements section is where you thank anyone who helped you significantly with the project or with the manuscript. For instance, you would thank your laboratory partners if they’re not authors on the paper, anyone who helped with the design of the experiment or the preparation of the paper. You might also include funding sources such as a Truman State University summer scholarship or a National Institutes of Health grant.
References
Most of the ideas presented in your paper are probably not exclusively yours. Therefore, you should cite other people’s work wherever appropriate. However, you do not need to cite information that is common knowledge or is exclusively your idea. The References section is a compilation of all citations made within the paper. It is not a bibliography and therefore should not list sources that are not directly referred to in the text.
The format of references varies amongst journals. For your chemistry laboratory reports, you should follow, by default, the ACS guidelines as outlined in The ACS Style Guide and Journal of the American Chemical Society, JACS (all examples given in this handout conform to JACS format). If your professor requires you to conform to a specific journal’s format, look at articles from that journal or refer to the journal’s “Instructions to Authors.” The specifications for most ACS journals are:
- 1) References should be compiled at the end of the paper in the References section.
- 2) References should be numbered in the order that they appear in the paper. For citations in the narrative, numbers should be superscripted and appear after the punctuation mark.
- y lines should be inserted between reference entries.
- 4) This section should be double spaced just like the rest of your paper.
- 5) A reference is only listed once in the References section. If multiple citations of the reference are made in the text, then the number corresponding to that reference is placed in the text each time. The common abbreviations used in footnotes and references (e. g., op. cit., ibid.) are not generally used in scientific writing.
Types of References
Articles. Journal articles are the primary source found in laboratory reports. An example is given below. Notice that the authors’ initials are given instead of the first and middle names. Also, there is no “and” before the last author’s name. Some journals require that the article’s title be included in the reference (check with your instructor to see if he/she wants you to use this style). When included, the article’s title should start with a capital letter but the other words in the title, unless they are proper nouns, should not be capitalized (see below). The journal title is abbreviated (click here for a list of the ACS abbreviations for common journals). Also, the year and the comma after the year are in bold. Lastly, the reference has inclusive pagination (first and last pages are given)
The following are examples of the same journal article with the first given in style where the article’s title is included in the reference, while the second is in the style where the article’s title is omitted.
- (1) Bergmann, U.; Glatzel, P.; deGroot, F.; Cramer, S. P. High resolution K capture X-Ray fluorescence spectroscopy: a new tool for chemical characterization. J. Am. Chem. Soc. 1999, 121, 4926-4927.
- (1) Bergmann, U.; Glatzel, P.; deGroot, F.; Cramer, S. P. J. Am. Chem. Soc. 1999, 121, 4926-4927.
Books. Books should be cited in the following manner:
- (2) Brünger, A. T. X-PLOR Manual, Version 3.1: A System for X-ray Crystallography and NMR; Yale University: New Haven, CT, 1990; pp 187-206.
- (3) Cheatham, T. E., III; Kollman, P. A. In Structure, Motion, Interaction, and Expression of Biological Macromolecules; Sarma, R. H. and Sarma, M. H., Eds, Adenine: New York, 1998; p. 99.
Computer Programs. Citations for computer programs vary. If a person in academia wrote the program, there is often a journal-article source. In other cases, the program is simply distributed by a company.
- Journal Article
-
- (4) Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33-38.
- Company Distribution
-
- (5) Case, D. A.; Pearlman, D. A.; Cladwell, J. W.; Cheatham, T. E.; Ross, W. S.; Simmerling, C. L.; Darden, T. A.; Merz, K. M.; Stanton, R. V.; Cheng, A. L.; Vincent, J. J.; Crowley, M.; Ferguson, D. M.; Radmer, R. J.; Seibel, G. L.; Singh, U. C.; Weiner, P. K.; Kollman, P. A. AMBER version 5.0; University of California: San Francisco, 1997.
-
- (6) Insight II; San Diego, CA: Molecular Simulations, 2000.
Websites. Journal articles are much preferred over websites. Websites are dynamic and are usually not peer reviewed. One of the only instances when a website is an acceptable reference is when it is referring to a database (however, an article is usually associated with the creation of the database). If you must use a website, the reference should include a title for the site, the author(s), year of last update and URL. It is unacceptable to use a website as a reference for scientific data or explanations of chemical processes.
-
- (7) Cheatham, T. E., III Simulation Protocol for Polynucleotides; 1998, http://www.amber.ucsf.edu/amber/tutorial/polyA–polyT.
Tables, Schemes and Figures
Tables, schemes and figures are all concise ways to convey your message. As you prepare these items for your report, remember to think of your reader. You want them to derive the maximum amount of information with the minimum amount of work. Pretend to be the reader and ask yourself, “Does this enhance my understanding?”, “Can I find everything?”, “Can I read it without being distracted?” Poorly prepared tables, schemes and figures will reflect badly on your science, and you as a scientist, so think carefully about these items as you prepare your report.
A table is a way to summarize data or ideas in a coherent, grid-like fashion. This is not simply output from a spreadsheet! You should prepare the table in a word-processor so that its formatting matches the rest of your report. In general, tables have no more than ten rows and columns to avoid overwhelming the reader. One common exception is in review articles (such as in Chemical Reviews) where an author is attempting to summarize results from an entire field. Another common exception is in the reporting of X-ray crystallography data. These tables have their own special formatting rules, and will not be discussed here.
Tables are referred to in the text as “Table #”. Tables, schemes and figures are labeled separately, with Arabic numbers, in the order they are referred to in the paper. Tables have a table caption, which in some journals appears above the table, while in others it appears below. In either case, the table caption is always on the same page as the table.
Don’t use lines or boxes in your table except where absolutely necessary. Use spaces between your columns instead (helpful hint: it is better to use your word processor’s table formatting tools than trying to get the columns to line up using tabs or spaces). All column or row headings should have clear subtitles and units if needed (usually in parentheses). Any numbers that are presented should have proper significant figures, and an indication of the error should be shown (click here to review how to report uncertainty in one’s data). An example table is given below.
-
- Table 1. Aminoacylation efficiency of duplexAla substrates containing base pair substitutions at the 2:71 position.
| 2:71 Base Pair | kcat/KM (relative)a | Fold decreaseb | -ΔΔG‡ (kcal/mol)c |
|---|---|---|---|
| G:C (wild-type) | 1 | 0 | |
| Watson-Crick Pur:Pyr Base Pairs | |||
| I:C | 0.51 | 1.9 | 0.39 |
| G:4HC | 0.25 | 3.9 | 0.81 |
| 2AA:U | 0.23 | 4.3 | 0.86 |
| 2AP:U | 0.18 | 5.6 | 1.0 |
-
- aValues reported are averages of at least three determinations with average standard deviations of ±26%.
-
- bFold decrease in kcat/KM is given relative to wild-type duplexAla.
-
- cΔΔG‡ is defined as RTln[(kcat/KM)variant/(kcat/KM)wild-type], where R=1.98272 cal/mol•K and T=298 K.
A scheme is usually a sequence of two or more chemical reactions that together summarize a synthesis. A scheme may also show the steps in a purification with each step or reaction giving the reactants, products, catalysts, and yields. A scheme that shows a chemical reaction may also show possible intermediates. Note that mechanisms are not usually conveyed using a scheme because they are more complicated and illustrate where electrons are proposed to move. Mechanisms are most often placed in a figure.
It is a common convention in a scheme to write a bold number underneath chemical species referred to in the text. Note that for the first occurrence of the bold number in the text, the chemical’s name is given, but after that only the bold number is used to identify it. This method of defining abbreviations for compounds can also be done in the experimental section, if there is no scheme. This is very useful when a compound’s name is long or complicated.
The one-step yield is usually written to the right of the equation, although it is also proper to write the yield under the arrow. Note also how the reaction conditions can be summarized (i. e., the first step below), which saves the reader from flipping to the experimental section for these details.
Each scheme also has a caption, which is included under the scheme. The caption should briefly summarize what is in the scheme. If the scheme is from another source, the reference to this source should appear at the end of the caption.
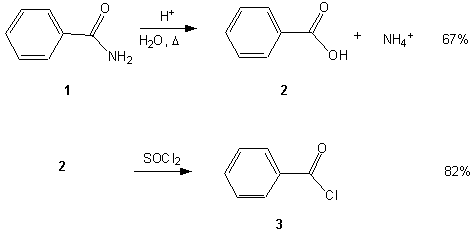
The following is an example of a scheme that might appear in a synthetic paper. The text below it shows how the scheme could be referred to in the body of the paper.
Benzamide (1) was refluxed under aqueous acidic conditions for 1 hour to yield benzoic acid (2). Acid (2) was then refluxed with SOCl2 to yield benzoyl chloride (3).
Sometimes a scheme may be used to illustrate a non-chemical process or how an instrument’s components are connected. These could also be presented as figures, and there is no definitive rule that will tell you when to use a scheme and when to use a figure. When in doubt, think of the reader and use the method that conveys the most information in the most easily understood format
Figures fall into two broad categories; those that are pictorial representations of concepts that are presented in the text, and those which summarize data. Again, it is critical to your report that your figures are clear, concise and readable, and that they support the arguments that you are making. Remember that you must refer to and discuss every figure in the text! If a figure is not mentioned, you don’t need it!
Figures that are pictorial representations of concepts usually appear in the Introduction, but it is also appropriate to include them in the Discussion. Use this type of figure to make your writing more concise (remember the conversion factor: 1 picture = 1 kword). Remember, humans are very visually oriented and we can grasp complex concepts presented as picture more easily then when they are presented in words or as mathematical formulae. Some examples of concept figures include:
-
- 1) An illustration of the deposition of metals onto a silicon wafer.
-
- 2) A diagram of the HIV life cycle.
-
- 3) A depiction of microwaves exciting water molecules.
-
- 4) A diagram illustrating the Frank-Condon principle.
-
- 5) A proposed organic mechanism.
Graphs are figures that present data. You use a graph when you have more data than will fit in a table. The general rules for preparing good figures for your notebook also apply in a laboratory report (click here to review graph preparation). Formatting tips: do not use colored backgrounds or gridlines, and do not draw a box around the graph.
You may find it more concise to combine all your data into one graph. For example, it may be appropriate to put six lines with absorbance as a function of time, with varying concentrations of a reactant on the same graph rather than constructing six different graphs. However, when doing this, be careful not to over-clutter the graph.
Standard curves should not be included in this section unless that was the primary goal of the experiment. They should be put in the Supporting Information.
Figures have figure captions compiled in the Figure Legend section, located on a separate page at the end of the paper. Journals chose this format because of typographical issues, and it has been retained despite its inconvenience to the reader. Each figure should appear on its own page in the order is it is discussed in the text. Figure captions appear in the Figure Legends section and do not appear on the same page as the figure. However, in the bottom, right-hand corner of the page the following identifying text appears:
- “First author’s last name et al., Figure number”
Figure Legends
All figure legends (captions) should be found in the section entitled “Figure Legends”. The format for a figure legend is usually: “Figure number” (italics and bold), a short title (followed by a period) and then a description of what is in the figure. All figure legends are compiled on the same page separated by a blank line. Be sure to define in the caption any symbols used in the figure, and note whether lines that pass through data points are fits, or “guides to the eye”.
- Example Figure Caption
- Figure 1. Nucleic acid bases. The chemical structures of (a) adenine, (b) guanine, (c) cytosine, and (d) thymine.
Supporting Information
This section (also known as Supplemental Material) is where you can include information that may be helpful, but not essential, for evaluation of your data. Items in this section may include calibration curves, and spectra (from which you extracted only one absorbance value for your analysis). Figures or tables of data whose contents were summarized in the text, or which were not critical to the conclusions, are also to be placed in the supporting information. An example of this type of material is the table of atom positions generated in an X-ray crystal structure.
- 1. Click here to obtain this file in PDF format. (link not yet active)
- 2. Click here for an example of a completed laboratory report.
- 3. The ACS Style Guide; 2nd ed.; Dodd, J. S., Ed.; American Chemical Society: Washington, D.C., 1997.
- 4. Booth, W. C.; Colomb; G. G.; Williams, J. M. The Craft of Research The University of Chicago Press: Chicago, IL, 1995.
- 5. Spector, T. J. Chem. Educ. 1994, 71, 47-50. Click here to view as a PDF file (Truman addresses only).
- 6a. Journal of the American Chemical Society Instructions for Authors, 2007.
- b. Inorganic Chemistry Instruction for Authors, 2007.
- c. Chemical Reviews Instructions for Authors, 2007.
- 7. Any non-English word should be italicized. This includes Greek and German words, and their abbreviations, that appear as part of chemical names (e. g., ortho-, meta-, para-, cis-, trans-, E-, Z-, alpha-, beta-, etc.). Also italicized are the condensed forms of secondary (sec-), tertiary (tert-), etc. The primary exception to the rule for italicizing non-English words are the Greek and Latin prefixes that denote numbers in chemical names (e. g., mono-, bi-, tri-, etc.). Some common Latin phrases that appear in scientific writing are vide infra (“see later”),vide supra (“see earlier”), et al. (abbreviation of et alia, Latin for “and others”), e. g. (from Latin exempli gratia, “for example”, not usually italicized) and i. e. (from Latin id est, “it is”, also not usually italicized). Other Latin phrases and abbreviations commonly used in footnotes and references (e. g., op. cit.) are not used in scientific writing.