Oxidation of Ethanol by Chromium(VI)
Adapted by J. M. McCormick
Last Update: January 17, 2012
Introduction
In acidic solution the dichromate ion will oxidize primary alcohols to aldehydes, which can be further oxidized in the presence of excess dichromate to carboxylic acids. Under the same conditions secondary alcohols are oxidized to ketones, which are not susceptible to oxidation by dichromate.1 Westheimer first proposed the mechanism shown in Fig. 1 for the oxidation of alcohols by dichromate ion in 1949.2,3 In the first step the dichromate ion is protonated to form chromic acid in a rapidly established equilibrium. The chromic acid then undergoes a rapid, reversible reaction with the alcohol to form a chromate ester, which then decomposes in the rate-determining step to form H2CrO3 and the aldehyde or ketone. There are subsequent steps in which H2CrO3 and various other chromium species react with the resulting carbonyl compound until all of the chromium is in the 3+ oxidation state. Fortunately, these reactions are fast, and will not complicate the kinetics that we wish to study.
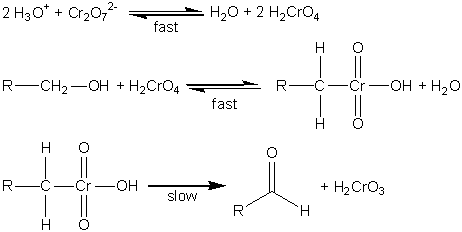
Figure 1. Proposed mechanism for the oxidation of alcohols to aldehydes (or ketones).2-4
In this exercise you will test the proposed mechanism by determining the rate law for the oxidation of ethanol by dichromate ion in acidic solution. The isolation method will be used with the alcohol’s concentration being much larger than the [Cr2O72-]. However, by varying the ethanol’s concentration you will be able to determine the order of the reaction with respect to ethanol. This is similar to the kinetics exercise you did in CHEM 120, and you are referred there for more information. One problem that must be overcome in this exercise is that both Cr2O72- (yellow) and the Cr3+ (green) strongly absorb light in the visible portion of the spectrum. Therefore, you will need to choose a wavelength at which the Cr2O72- absorbs, but the Cr3+ does not. Even then you will need to measure the absorbance at an infinite time (A8) to correct for any residual absorbance at this wavelength.
Experimental
Prepare a stock solution of 3.9 M H2SO4 from concentrated H2SO4. Precisely prepare a 0.0196 M K2Cr2O7 solution from the solid using distilled water. When preparing the latter solution, take into account how much of it you will use for this exercise and minimize the amount of waste. CAUTION! Concentrated sulfuric acid can cause severe burns and chromium(VI) is a known carcinogen.
Kinetics data will be obtained by measuring the decrease in the chromium(VI) species’ absorbance as a function of time. The instrument that will be used is an Ocean Optics USB2000 Vis-NIR spectrometer with a water-jacketed cell holder so that the sample’s temperature may be held constant by means of an external water bath. Allow sufficient time for the spectrometer to warm up and the water bath to attain equilibrium before proceeding with a kinetics run. Be sure that you know how to take wavelength scans and perform kinetics measurements with the spectrometer before coming to lab. Note that, by convention, kinetics data are obtained at 25 °C. Therefore, set the water bath to 25 °C initially and be sure water is circulating through the jacketed cell holder.
Prepare a solution of the dichromate solution by transferring 1 mL of the 0.0196 M K2Cr2O7solution into 10 mL of the 3.9 M H2SO4 solution. Mix well and obtain the absorption spectrum of this solution (save it for later use). Prepare a solution of CrCl3·6H2O of approximately the same concentration in 3.9 M H2SO4, obtain its spectrum (save it, too). Determine the wavelength at which you will follow the reaction and record the absorbance of the dichromate solution at this wavelength (A0). Set up the spectrometer’s kinetics routine to obtain data at least once a minute at this wavelength for 10 min. Don’t forget to set a delay time to account for mixing of the reagents (1 min, or less, should be appropriate). You may find that these initial settings are not adequate, and you may change these parameters as needed to optimize data collection.
To start a kinetics run prepare the chromic acid solution by mixing 1 mL of the 0.0196 M dichromate solution and 10 mL of the 3.9 M H2SO4 solution in a small beaker. Add to this 10.0 µL of absolute ethanol, starting the count down on the spectrometer at the same time. Swirl the solution in the beaker to assure complete mixing, transfer the solution to a cuvette and place the cuvette in the spectrometer. Once the data collection has stopped, remove the solution from the cuvette and place it in a safe place. After an hour, measure the absorbance at the same wavelength that you monitored for the kinetics run (this is A8). As you prepare for the second run, graph the data from your first run using the integrated rate laws (at this point don’t worry about A8) and critically evaluate the acquisition parameters. Change the acquisition parameters as needed.
While you are waiting for the infinite time on the first run, repeat the kinetics runs twice more (you may need to arrange with the instructor how you will obtain A8 for your last run) examining the data from the previous run while the next data is being acquired. Be sure that your data is consistent and makes sense. Is A8 so small that it may be ignored?
With the data from the first run and the integrated rate laws, determine the order of the oxidation with respect to dichromate.
Once you have determined the order with respect to dichromate, vary the ethanol concentration. From these data determine the order with respect ethanol and the rate constant for the reaction.
Determine the activation energy for the slow step of the reaction by varying the temperature with whichever reactant concentrations gave you the best results. Note that at least three runs must be made at each temperature and that data at a minimum of three additional temperatures must be obtained.
Derive the rate law from the mechanism shown in Fig. 1.
Results
You will need to present an example of each of the integrated rate law graphs demonstrating the order with respect to Cr2O72- (i. e., a zeroth order graph and a first order graph and a second order graph), and the graph that you prepared to demonstrate the order with respect to the ethanol. Include the Arrhenius plot from which you determined the activation energy. Don’t forget to calculate uncertainties on the rate constant and the activation energy.
Conclusions
Discuss whether your results are consistent with the proposed mechanism and with the previously reported results.
1. Pavia, D. L.; Lampman, G. M.; Kriz, Jr., G. S. Introduction to Organic Laboratory Techniques: a Contemporary Approach, 2nd Ed.; Saunders: Philadelphia, PA; 1982, pp. 194-200.
2. Westheimer, F. H. Chem. Rev. 1949, 45, 419. Click here to obtain this article as a PDF file (Truman addresses and Chem. Rev. subscribers only).
3. Westheimer, F. H. and Nicolaides, N. J. Am. Chem. Soc. 1949, 71, 25. Click here to obtain this article as a PDF file (Truman addresses and J. Am. Chem. Soc. subscribers only).
4. Lanes, R. M.; Lee, D. G. J. Chem. Educ. 1968, 45, 269. Click here to obtain this article as a PDF file (Truman addresses and J. Chem. Educ. subscribers only).
