Synthesis of a Porphyrin and its Metal Complex
Last Update: August 29, 2011
Procedure is identical to that of Girolami et al.1 with the exception that you can choose a different aldehyde from those in stock and you can choose any metal you want.
Important changes to the procedure are: 1) use TLC to assess the purity of the ligand before making any metal complexes; purify if necessary, 2) you do not need to shake the porphyrin solution in CDCl3 with H2O, 3) use TLC to determine the purity and the stability of your metal complex before attempting to purify it by column chromatography.
We have the aldehydes shown below available for use in this synthesis.
 | 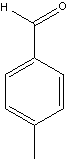 | 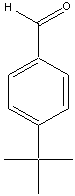 |
| Bezaldehyde | Tolualdehyde | 4-tButylbenzaldehyde |
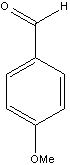 |  |  |
| p-Anisaldehyde | Valeraldehyde | Heptanal |
Reference
- 1. Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J. Synthesis and Techniques in Inorganic Chemistry: A Laboratory Manual 3rd Ed.; University Science: Sausalito, CA, 1999, Experiment 23.
