Bromination of Acetone
Adapted from an Exercise developed by the Physical Chemists at the University of Kansas by J. M. McCormick
Last Update: November 3, 2013
Introduction
Under acidic conditions ketones react with halogens to give substitution at the alpha-carbon, as shown in Fig. 1 for the reaction of Br2 for acetone. Shown in Fig. 2 is a proposed mechanism for this reaction.1 In this exercise you will test whether this proposed mechanism is consistent with the experimental rate law by first experimentally determining the rate law using the isolation method (click here to review kinetics) and deriving the rate law predicted by this mechanism. In addition, because this mechanism involves the breaking of a C-H bond it is expected that it should display a kinetic isotope effect when D is substituted for H. Therefore, the rate of bromination of deuterated acetone will be measured to determine if there is a kinetic isotope effect, and thus provide further evidence for the proposed mechanism.
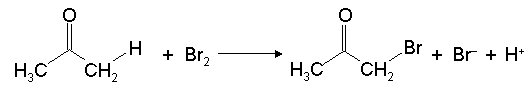
Figure 1. Reaction of Br2 with acetone to give a-bromoacetone, Br– and H+.1
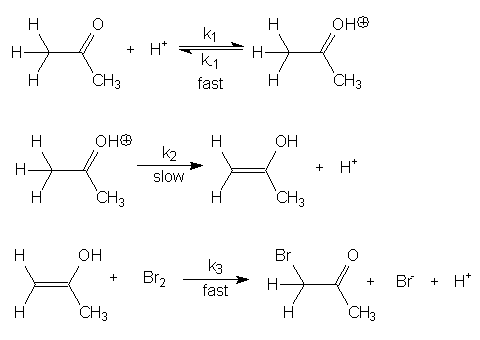
Figure 2. Proposed mechanism for the reaction of Br2 with acetone.1
Experimental
CAUTION! Concentrated HCl is corrosive. Acetone is flammable and volatile. Br2 is a corrosive strong oxidant and very toxic. Avoid breathing Br2 fumes. Work with the concentrated bromine water solution only in the hood. The products of these reactions are lachrymators and moderately toxic. Handle all solutions with care and dispose of all waste properly.
Note that Br2 absorbs in the UV. Therefore, you will only be able to use either an Ocean Optics spectrometer whose range is set for the UV (the Ocean Optics spectrometers in MG 1026 are not set for the UV) or the Cary 50. Before preparing the solutions, you will need to warm up the spectrometer.
Refer to the schemes for the procedure for determining the order with respect to each reactant. Note that you will prepare the solutions on the left and right hand side of each scheme and then mix these together, as indicated, to give the solutions that you will make the kinetics measurements on. These solutions are in the center of each scheme in a red box. It is suggested that you first determine the order with respect to Br2 (Scheme 1).
Use distilled water to prepare all solutions. Prepare a 1 M aqueous HCl stock solution from concentrated HCl, and a 4 M aqueous acetone stock solution. The concentration of neither solution needs to be precise, but you should have at least two or three significant figures for each. Note that the concentration of the acetone stock solution will change appreciably if it is left uncapped.
As the Br2 solution is colored (extinction coefficient at 400 nm, e400, is 160. M-1·cm-1), you will follow the reaction by the disappearance of the Br2 absorbance at 400 nm (A400). A saturated solution of Br2 in distilled water (bromine water) will be provided. The [Br2] in the saturated solution is approximately 0.16 M, but it will change with temperature. Therefore, the [Br2] must be determined at the start of each laboratory period. To find the [Br2], first take 2.00 ml of the stock solution and dilute to 100.0 ml with water in a hood. Measure the absorption spectrum of this diluted solution and from A400 determine the [Br2] using Beer’s Law.2 The dilutions in Schemes 1-3 assume a [Br2] of 0.16 M, and as such you will need to work out the actual bromine concentrations based on your absorbance measurement.
To perform a kinetics run, the spectrometer (for Ocean Optics spectrometers, click here; for the Cary 50, click here) to record data at the maximum Br2 absorbance and make any necessary background/baseline corrections. You will need to set the spectrometer’s software to acquire the data at some set interval and to delay the start of data acquisition long enough for you to mix the reagents and place the cuvette in the sample holder. This may require some trial and error. It is important that you take as much data as possible right after the reagents are mixed. The absorbance change will deviate from ideal behavior as the reaction proceeds because of further halogenation reactions of the bromoacetone. Only data within the first one to two minutes after mixing may be useable and you may observe significant deviations after this time.
To start the kinetics run, place the two reagents to be mixed, as per the particular scheme you are following, in separate beakers. Pour one into the other and start the software countdown when they are half mixed. Swirl the solution for a few seconds to mix the reagents well and quickly pour the solution from the beaker into a cuvette. Place the cuvette in the cell compartment of the spectrophotometer before the delay time is up. Once you have finished a run you should immediately make a graph of your data to check whether you need to change the delay time, or the sampling frequency. Take at least one measurement at a sufficiently long time to represent the residual absorbance at t= 8. Based on this result you will know whether you need to make absorbance readings at t= 8 for all of your kinetics runs. Make at least three runs for each different starting condition.
After you have determined the order with respect to each reactant, determine the rate of reaction for d6-acetone (where all 1H are replaced with 2H = D). Determine whether there is any kinetic isotope effect (kH/kD, where the kH and kD are the rate constants for the reaction with and without deuterium, respectively).4 Deuterated acetone is fairly expensive, so carefully plan which set of reaction conditions given in the schemes will give you the best results. You are to prepare only enough of the 4 M d6-acetone to perform the experiments.
Results
Graph the raw kinetics data in Excel as described in the kinetics review document. Extract the orders with respect to each reactant, rates and rate constants from a best fit of the data. Include a representative example of each graph in the results section of your report.
Report your results (order with respect to each reactant, the overall rate constant and kH/kD) including uncertainties. Propagate the error in each slope and rate of reaction through to the order of reaction and rate constant. A detailed propagation error for the dilutions is not necessary because of the large number of dilutions involved. However, make sensible estimates of these errors where appropriate. Note that the order of reaction is usually has integer or half-integer values. So, a value of 0.91±0.03, for example, may be taken as being equal to one.
Derive the rate law for the mechanism given in Fig. 2, assuming that the first equilibrium is rapidly established (K = k1/k-1), the second step (k2) is slow and the final step (k3) is fast.
Conclusions
In your Discussion be sure to address the following: 1) whether this mechanism is consistent with your experimentally determined rate law consistent; 2) if the rate law is consistent with the mechanism, does it necessarily prove the mechanism and the structures of the intermediates shown? 3) what rate constants in the mechanism does the measured rate constant correspond to? 4) what is the role of H+ in this reaction? 5) is your measured kinetic isotope effect consistent with the mechanism?
1. Solomons, T. W. G. Organic Chemistry, 4th Ed.; Wiley: New York, 1988, p. 788.
2. Beer’s law states that the absorbance, A, is directly proportional to the extinction coefficient, e, the concentration, C, and the pathlength, b, or A = e·b·C.3
3. Skoog, D. A.; West, D. M. and Holler, F. J. Fundamentals of Analytical Chemistry, 5th Ed.; Saunders College Publishing: New York, 1988, pp. 464-469.
4. Sykes, P. A Guidebook to Mechanism in Organic Chemistry, 5th Ed.; Longman: New York, 1981, p. 46-48.